Probiotics have gained in popularity and use over recent years, yet many of us are unsure of the exact benefits that supplementing with a probiotic blend can have; particularly when it comes to athletic performance.
In our latest X-Change paper, written by leading researchers on probiotics and gastrointestinal health in athletes Dr Jamie Pugh and Dr Justin Roberts, we explore the role that probiotics play in the digestive system, specifically looking at supplementation's effect on digestive function, the immune system and athletic performance.
Read the full X-Change article here or download the PDF via the button below.
Practical Implications
- Probiotic supplements contain live microorganisms or bacteria, identified as having potential health benefits. They vary with regard to the genus, species, and strains included, as well as the dose (or colony forming units – CFU) of each product.
- While there are some shared characteristics of probiotics, there are many strain-specific mechanisms. Use of specific probiotic formulas for targeted health outcomes should be based upon an evidence-based approach utlising relevant research trials.
- In both the general and athletic population, there is good evidence to suggest that probiotics reduce the incidence and/or the severity of upper respiratory tract infection and infectious diarrhoea. Athletes should therefore consider probiotic use when there is an increased risk of infection (e.g., international travel and/or during anti-biotic treatment).
- Probiotics may attenuate exercise-induced gastrointestinal symptoms, although other nutritional factors using a food-first approach should be initially considered.
The Gut Glossary – an ever-increasing number of terms
Microbiota and Microbiome - The gut microbiota is the diverse ecosystem consisting of bacteria, archaea, viruses, protists and fungal communities that reside in the human gut. Microbiome refers to the collection of genomes from all microorganisms in a particular environment.
Probiotics - Live microorganisms which, when administered in adequate amounts, confer a health benefit on the host (these tend to be described as ‘good bacteria’).
Prebiotics - A substrate that is selectively utilized by host microorganisms conferring a health benefit on the host (compounds that are generally undigested and not absorbed by humans that then provide food for the bacteria). Common examples are inulin, fructo oligosaccharides [FOS], and galactooligosaccharides [GOS]).
Synbiotics - A product that beneficially affects the host in improving the survival and implantation of live microbial dietary supplements in the gastrointestinal tract by selectively stimulating the growth and/or activating the metabolism of one or a limited number of health-promoting bacteria (probiotic + prebiotic). The amount of prebiotic in these commercial products tends to be much, much lower than that found in prebiotic supplements.
Postbiotics - Bioactive components produced by beneficial bacteria (through a natural fermentation process) which have biological activity in the gut, e.g., short-chain fatty acids.
Background
Probiotics (derived from a Greek word meaning “for life”) are defined as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host (Hill et al. 2014). Unlike other dietary supplements, probiotic preparations contain live, viable, defined microorganisms (bacteria). It has been over a century since the idea that our health can be influenced by the bacteria residing in our intestinal tract (our microbiome) and can be improved with the consumption of soured milk-containing groups of lactic acid producing bacteria (Mackowiak 2013). Since then, our understanding of the role the microbiome plays in our wider health, and thus the potential for probiotic supplementation, has, and continues, to grow. Probiotics have been studied not only for their benefits to the gastrointestinal (GI) tract, but also for their potential to improve immune function, metabolic health, mood and cognition, and athletic performance (Jäger et al. 2019). It has long been known that there is a role of the gut as a major organ aiding immune function in humans (Doe 1989) (something discussed in the NutritionX article on ‘Science behind Glutamine’).
Basics of Probiotic
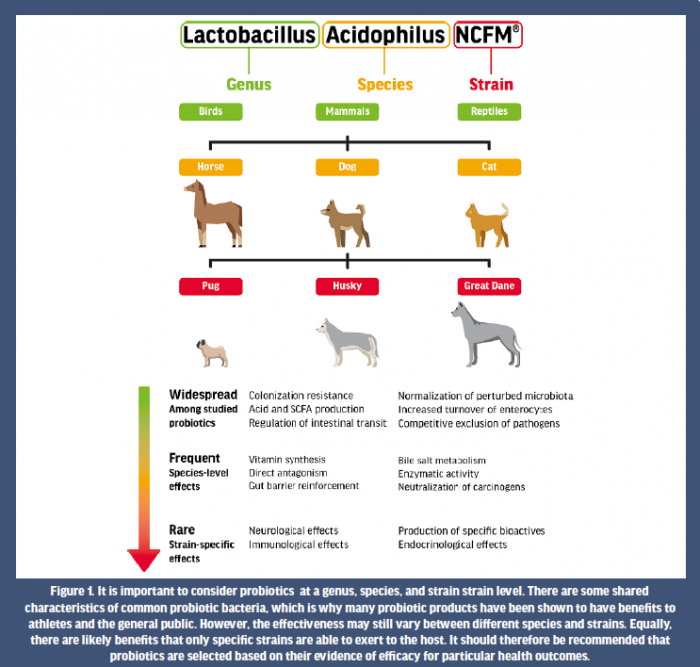
More than 500 different bacterial species reside in the adult gastrointestinal tract (Pham et al. 2008). Some microbes are considered beneficial to the human host, while others can be pathogenic. For bacteria to be considered a probiotic, they must possess certain characteristics. Probiotic organisms must be able to withstand passage through the gastrointestinal tract (i.e., survive acid- and bile induced degradation), they must be safe and effective in humans, remain viable for the shelf life of the product, and not have pathogenic properties (Senok et al. 2005). When referring to a probiotic, the genus, species, and strain of each live microorganism must be considered, as well as the total estimated quantity of each probiotic strain at the end of the product’s shelf life, as measured by colony forming units (CFU) or live cells. The strains most commonly used typically belong to the genera Lactobacillus, Bifidobacterium, Enterococcus, or Propionibacterium or certain yeasts such as Saccharomyces boulardii. Even though they may exist within the same species, two different probiotic strains possess unique transcriptomes with different mechanisms of action and potential benefits (McFarland et al. 2018). Therefore, while probiotics may share some common benefits, there is a clear strain specificity that should also be considered e.g. all breeds of dogs can bark, but some louder than others, and some breeds also have unique skills that others don’t have (Allen et al. 2017; Sanchez et al. 2017) (Fig 1). This generally results in confusion for both consumers and health practitioners, particularly when a range of commercial probiotic products are available on the market.
Probiotics are commonly available as concentrated capsules, as a powder, or in various dairy or fermented food products. Although fermented foods, such as sauerkraut or kimchi, contain live microbes, they are currently not classified as probiotics, as those products have not currently been sufficiently studied for their health benefit as stipulated by the definition of probiotics, or characterised regarding the number and taxonomy of bacterial strains they contain. Probiotic supplements may contain one bacterial strain or multiple strains from different species and doses of probiotics have ranged from 1-100 billion colony forming units (CFU)/g per day (Table 1). The effective dose of probiotics is influenced by a multitude of variables, including health endpoint, the specific species used, and method of delivery (Ouwehand 2017). There is also a paucity of data to have investigated any dose-response effect that might exist. These factors make it difficult to generalise one optimal dose for probiotic effects. The most common dose of commercial probiotics is likely around 7-25 billion CFU/day and in a small number of studies, these have been shown to be as effective as >100 billion CFU/day in outcomes such as colonic transit or symptom improvement in irritable bowel syndrome (IBS) (Ouwehand 2017).
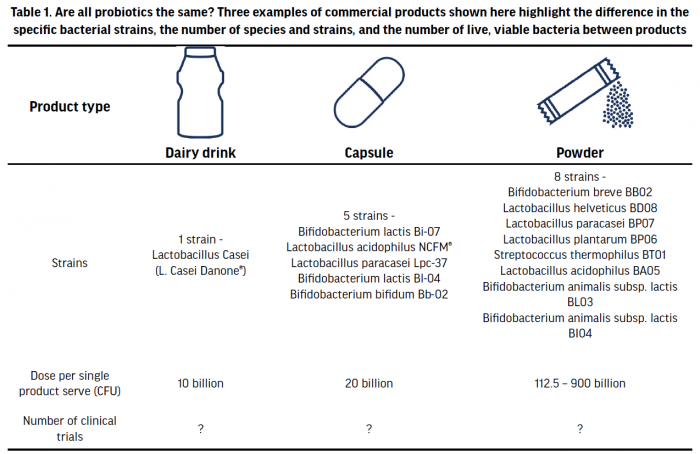
The number of clinical trials involving commercial probiotic supplements can vary greatly between products, and this information can be difficult to ascertain (leading to reduced clarity around scientific robustness and/or efficacy). Additionally, for those supplements that have been used in clinical trials, most studies typically only compare specific probiotic supplements to an inert placebo and not a different probiotic. Consequently, it is difficult to directly compare the effectiveness of one product over another. When selecting a probiotic, specific strains and doses should be selected that have been shown to be efficacious in producing a specific benefit (e.g., reduction in anti-biotic associated diarrhoea, reduced upper respiratory tract infection (URTI), etc.).
Mode of Action
It is known that ingestion of probiotic strains is not associated with long-term colonisation and survival in the host, as probiotic strains are only retained for days or weeks after discontinuation of ingestion (Senok et al. 2005). Therefore, their effects appear to be transient, and continuous long-term intake is necessary. Given that different strains and product formulations exist, there is no single answer as to how they work. However, there viable proposed mechanisms by which probiotics potentially exert their benefits. In general, probiotics do not permanently colonize the GI tract; however, supplementation may affect the composition, functionality, and metabolism of the resident microbiota (Derrien & van Hylckama Vlieg, 2015). These mechanisms can be local to the GI tract given that probiotic bacteria can directly interact with the mucus layer, epithelial layer, and gut-associated lymphoid tissue of the GI tract (Hill et al. 2014) (Fig 2A). Interestingly, some probiotic strains also demonstrate the ability to confer effects distant to the site of administration, such as via the synthesis of soluble factors (e.g. short-chain fatty acids) that themselves can have systemic beneficial effects for the host, or even via the production of neurochemicals which can have effects upon the brain (Mörkl et al. 2020; Sanchez et al. 2017) (Fig 2B). Although difficult to study, it is likely that most probiotics exert some common benefits. For example, different probiotic strains have been found to inhibit the growth of pathogens such as Escherichia coli and Salmonella, although to varying degrees and via strain specific mechanisms (Muñoz-Quezada et al. 2013). Due to the difficulties in taking human biological samples from the GI tract, many clinical studies must take a ‘black box’ approach. Collecting and analysing faecal samples can be logistically challenging and so these data are often not included in studies. Equally, even if a study incorporates faecal samples, these only reflect the very distal end of the colon, and do not help us understand what changes might be occurring all throughout the gut (Zmora et al. 2018). Probiotics then, are often studies as the “input” and a health outcome is often seen as the “output” with many studies not fully describing the intervening mechanisms (Jäger et al., 2019).
Benefits of probiotics to athletes
Probiotics have been studied and shown to be beneficial in a number of conditions including; infectious diarrhea, antibiotic associated diarrhea, lactose intolerance, GI symptoms, and symptoms of irritable bowel syndrome (George Kerry et al. 2018). More recently, there has also been interest in the role of probiotics in athletic performance, recovery from exercise, adaptation to training, changes to body composition, and mood and cognition (Jäger et al. 2019). It is difficult to make clear conclusions for some of these areas of research at this point though due to the small number of studies and the large variation in study design and different probiotic strains used. For example, there is limited and contrasting data in regard to athletic performance. Where there is perhaps the most research to date is in the areas of immune function and GI associated benefits.
Immune function
The beneficial effect of probiotic supplementation on immune function modulation is perhaps the most extensively researched for athletes. The mucosal lining of the GI tract represents the first line of defence against invading pathogens and is an important interface with the host immune system. Probiotics may regulate the immune response by modulating signalling pathways that lead to enhanced mucus or defensin production, or by preventing apoptosis or they may increase tight junction function (Bron et al. 2011). Particular strains of bacteria (such as those belonging to the genera Lactobacillus and Bifidobacteria) influence the gene expression of mucins, Toll-like receptors, caspases, nuclear factor-κB, and interleukins which promotes an anti-inflammatory response (Plaza Diaz et al. 2014).
Focusing on the real-world effects of probiotic supplementation, on the prevalence or severity of infectious illnesses, a Cochrane review of nonathlete participants identified 12 trials, involving 3720 participants. This review found that probiotics were better than a placebo in reducing the number of participants experiencing episodes of acute URTI by 47% and the duration of an episode of acute URTI by 1.89 days, as well as reducing antibiotic prescription rates (Hao et al. 2015). A further review paper identified 22 studies which assessed the effect of probiotics in athletes on outcomes related to the immune system. Of these, 14 reported significant improvement, whereas 8 reported no effects yet, importantly, no negative effects were reported (Jäger et al. 2019). For example, in a group of 56 well trained endurance athletes studied over 16 weeks during the winter, those that supplemented with 6.5 billion CFU/day of Lactobacillus casei Shirota daily, reported half the number of URTI episodes (1.2 ± 1.0) compared with those supplementing with a placebo (2.1 ± 1.2) (Gleeson et al. 2011). A study of 39 elite athletes during 14 weeks of winter training found that those supplementing with 20 billion CFU/day of Lactobacillus helveticus Lafti L10 daily, reported a shortened duration of URTI episodes (7.3 ± 2.9 vs. 10.6 ± 4.7 days) and fewer URTI symptoms (4.9 ± 1.9 vs. 6.9 ± 1.2) compared with a placebo (Michalickova et al. 2016). Although not definitive, the evidence for probiotics in reducing the burden of URTI for athletes (and the lack of evidence for harm) supports their use, especially during periods of increased URTI risk, such as international travel and competition or during winter periods.
Gastrointestinal health
Given that infectious GI illnesses represent one of the most common illnesses reported during major international competition (Engebretsen et al. 2013) combined with the high use of antibiotics in sport (Rossi et al. 2021; Tscholl et al. 2015), acute GIrelated illnesses are a particular area of interest for athletes. In the general population, there is evidence to suggest that probiotics are beneficial for treating or preventing acute infectious diarrhoea (Allen et al. 2010) and traveller’s diarrhoea (Bae 2018). Athletes use oral antibiotics twice as frequently as age-matched controls (2.7% vs 1.3%) (Alaranta et al. 2008) and one of the common side effects associated with their use is antibiotic-associated diarrhoea. A meta-analysis of 63 studies, including 11 811 participants, found that probiotic use was associated with a reduced relative risk (0.58) of developing antibiotic associated diarrhoea compared with a control group (Hempel et al. 2012). If probiotics are not regularly consumed, it may then at least be of benefit to consider supplementation prior to and during international travel or antibiotic use, and at the onset of acute infectious diarrhoea.
While acute infection is relatively uncommon, a significant number of athletes may experience chronic, less severe GI-related symptoms either at rest or during exercise. Cochrane reviews and meta-analyses have shown that probiotics have beneficial effects on symptoms of irritable bowel syndrome (Ford et al. 2014; Parker et al. 2018). While the underlying cause of symptoms may be different, many athletes experience similar functional GI symptoms. For example, from a sample of 249 elite athletes, resting symptoms of abdominal bloating and flatulence were reported in 48% and 44% of participants, respectively (Pugh et al. 2018a). Exercise, particularly endurance exercise, also causes a homeostatic challenge to the GI tract and may result in the manifestation of GI distress. Indeed, as many as 25% of all marathon runners report symptoms during training and competition (Pugh et al. 2018b). The incidence of GI symptoms is even higher still in ultra endurance events where GI symptoms are often the leading cause of race disruption or dropout (Stuempfle and Hoffman 2015; Stuempfle et al. 2016). These exercise-associated symptoms may then impact training, competition performance and/or quality of life depending on their nature and severity. A small number of studies have evaluated the effect of probiotics on GI symptoms in athletes, although methodological issues persist with many studies reporting only the frequency or duration of symptoms, and not the severity. While there are some studies showing no effect of probiotic supplementation (Kekkonen et al. 2007; West et al. 2014), GI symptoms were not the primary outcome. Studies that have specifically investigated GI symptoms as a primary outcome measure have shown attenuations, although not complete alleviation, of symptoms reported by endurance athletes. In marathon runners, supplementation with 25 billion CFU/day of Lactobacillus acidophilus (CUL60 and CUL21), Bifidobacterium bifidum (CUL20), and Bifidobacterium animalis subs p. Lactis (CUL34) (LAB4) led to a reduction in the number of training days in which athletes suffered lower GI symptoms (Pugh et al. 2019; Roberts et al. 2016). Supplementing with this same multi strain probiotic was also associated with less severe GI symptoms experienced during the end stages of a marathon race compared to placebo (Pugh et al. 2019). It should be noted that in both studies, symptoms were still present, alluding to the fact that symptoms are multifaceted and individual. Probiotic strains reported to reduce the severity of GI symptoms may then be considered by athletes with exercise associated symptoms, although other nutritional strategies are likely also needed for complete therapy. If symptoms persist, clinical referral may be needed to rule out a potential underlying pathology.
Athletic Performance
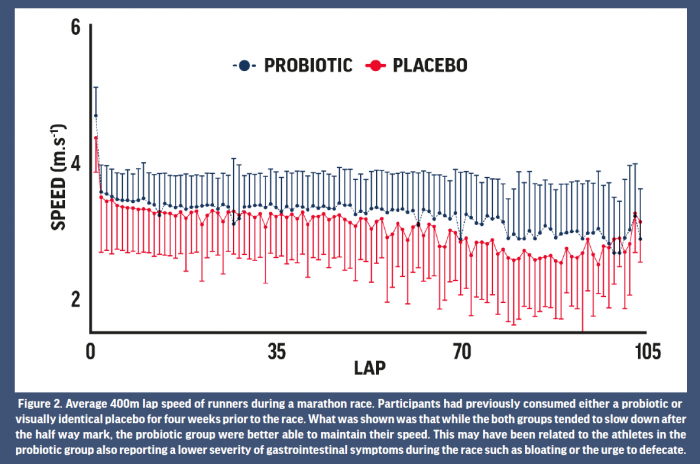
Outcomes related to athletic performance remain one of the most difficult of all measures to effectively capture in scientific studies. Relatedly, research pertaining to the effects of probiotics on athletic performance to date has reported mixed results. Both null and positive findings have been reported for aerobic capacity following a period of supplementation, although a large range of methodologies have been reported. For example, 4 weeks of supplementation of a multiple strain probiotic improved time to fatigue in ten male runners while running on a treadmill in the heat (Shing et al., 2014). Unfortunately, the authors could not elucidate a specific mechanism for this improvement. Possible mechanisms suggested have included maintenance of the GI barrier, alterations in the sensation to fatigue, and benefits to exercise metabolism (Jäger et al. 2019). While a direct ergogenic role of probiotics on athletic performance remains speculative, athletic success is well-known to be associated with consistency in training and less time lost to injury and illness. Given the evidence for probiotics to reduce the risk of infectious illness, as described above, this is one possible mechanism for improvements in performance over time. In thirty three trained athletes, during 12 weeks of winter training the training load (hours per week) was higher in those who supplemented with probiotic compared with the placebo group which the authors attributed to reduction of risk of illness in the probiotic group (Strasser et al. 2016). There is also some evidence that probiotic supplementation, paired with protein, can accelerate recovery from damaging bouts of exercise (Jäger et al. 2016). Studies that have investigated the effect of probiotics on real-world endurance races have typically reported null effects (Marshall et al. 2017; Roberts et al. 2016), although perhaps this is to be expected given the large number of factors that contribute to performance in these events. GI symptoms during such races can affect performance though and may be an indirect mechanism by which probiotics could improve performance. In ultramarathon competitors, GI symptoms have been reported to negatively impact race performance in 44% of runners, while symptoms were also the largest contributing factor to non-completion of a race (Stuempfle and Hoffman 2015). During a marathon race in which GI symptoms and running pace were monitored throughout, those that had supplemented with probiotics in the 4 weeks prior to the race developed less severe GI symptoms during the latter stages of the race and this was associated a better maintenance of running pace (Pugh et al. 2019) (Fig 2). While several studies have found no effect of probiotics on endurance performance, there may be indirect mechanisms (reduction in missed training, attenuation of GI symptoms) by which probiotics could lead to improvements in athletic performance over time. Taken together, whilst the evidence for probiotics improving performance is, at best, equivocal, this is a promising area of research that needs to be fully explored with mechanisms identified.
Future Directions
There is a clear need for more research directly comparing the effectiveness of different commercial strains, as well as a better understanding of any dose response effect, which are lacking in both athletic and general population studies. Regarding further benefits of probiotics specifically for athletes, there is some evidence to suggest that supplementation can have other impacts for athletes. Preliminary data has shown that probiotic supplementation increases absorption of amino acids from plant protein (Jäger et al. 2020) and increases exogenous carbohydrate absorption and oxidation during exercise (Pugh et al. 2020). There is growing evidence that probiotics impact psychological states and have benefits on mood or stress, anxiety, and depression (Mörkl et al. 2020) but data there is currently no data in athletes. Limited data has also shown probiotics to reduce exercise-induced muscle damage and increased the rate of recovery (Jäger et al. 2016). Future studies are needed to confirm these early data by replication and, in many instances, more robust study design. Given the potential for strain-specificity, it is important to understand the exact mechanism for many of these new areas of interest. This should help.
Summary
Athletes should consider the context and need for probiotic supplementation, based on the available evidence. While there are shared, proposed mechanisms by which probiotics can exert beneficial effects, athletes should still look to select commercial strains and doses that have been shown to be effective for a particular outcome (e.g., reducing the risk of traveller’s diarrhoea during international travel). Given the transient effects of probiotics, it is important to consume the supplement continuously during these time periods. If an athlete chooses to consume a regular/ daily supplement for general GI/immune health, it may still be advantageous to select one that has been used in some clinical or research trials.
References
- Alaranta A, Alaranta H, Helenius I (2008) Use of prescription drugs in athletes Sports medicine (Auckland, NZ) 38:449-463 doi:10.2165/00007256-200838060-00002
- Allen AP, Clarke G, Cryan JF, Quigley EMM, Dinan TG (2017) Bifidobacterium infantis 35624 and other probiotics in the management of irritable bowel syndrome. Strain specificity, symptoms, and mechanisms Curr Med Res Opin 33:1349-1351 doi:10.1080/03007995.2017.1322571
- Allen SJ, Martinez EG, Gregorio GV, Dans LF (2010) Probiotics for treating acute infectious diarrhoea The Cochrane database of systematic reviews 2010:Cd003048 doi:10.1002/14651858.CD003048.pub3
- Bae JM (2018) Prophylactic efficacy of probiotics on travelers' diarrhea: an adaptive meta-analysis of randomized controlled trials Epidemiology and health 40:e2018043 doi:10.4178/epih.e2018043
- Bron PA, van Baarlen P, Kleerebezem M (2011) Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa Nat Rev Microbiol 10:66-78 doi:10.1038/nrmicro2690
- Doe WF (1989) The intestinal immune system Gut 30:1679-1685 doi:10.1136/gut.30.12.1679 Engebretsen L et al. (2013) Sports injuries and illnesses during the London Summer Olympic Games 2012 British journal of sports medicine 47:407-414 doi:10.1136/bjsports 2013-092380
- Ford AC et al. (2014) Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis Am J Gastroenterol 109:1547-1561; quiz 1546, 1562 doi:10.1038/ajg.2014.202
- George Kerry R, Patra JK, Gouda S, Park Y, Shin HS, Das G (2018) Benefaction of probiotics for human health: A review Journal of food and drug analysis 26:927-939 doi:10.1016/j.jfda.2018.01.002
- Gleeson M, Bishop NC, Oliveira M, Tauler P (2011) Daily probiotic's (Lactobacillus casei Shirota) reduction of infection incidence in athletes International journal of sport nutrition and exercise metabolism 21:55-64 doi:10.1123/ijsnem.21.1.55
- Hao Q, Dong BR, Wu T (2015) Probiotics for preventing acute upper respiratory tract infections The Cochrane database of systematic reviews:Cd006895 doi:10.1002/14651858.CD006895.pub3
- Hempel S et al. (2012) Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta analysis Jama 307:1959-1969 doi:10.1001/jama.2012.3507
- Hill C et al. (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic Nat Rev Gastroenterol Hepatol 11:506-514 doi:10.1038/nrgastro.2014.66
- Jäger R et al. (2019) International Society of Sports Nutrition Position Stand: Probiotics Journal of the International Society of Sports Nutrition 16:62 doi:10.1186/s12970-019-0329-0
- Jäger R et al. (2016) Probiotic Bacillus coagulans GBI-30, 6086 reduces exercise-induced muscle damage and increases recovery Peer J 4:e2276 doi:10.7717/peerj.2276
- Jäger R et al. (2020) Probiotic Administration Increases Amino Acid Absorption from Plant Protein: a Placebo-Controlled, Randomized, Double-Blind, Multicenter, Crossover Study Probiotics Antimicrob Proteins 12:1330-1339 doi:10.1007/s12602-020-09656-5
- Kekkonen RA, Vasankari TJ, Vuorimaa T, Haahtela T, Julkunen I, Korpela R (2007) The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners Int J Sport Nutr Exerc Metab 17:352-363
- Mackowiak PA (2013) Recycling metchnikoff: probiotics, the intestinal microbiome and the quest for long life Frontiers in public health 1:52 doi:10.3389/fpubh.2013.00052
- Marshall H, Chrismas BCR, Suckling CA, Roberts JD, Foster J, Taylor L (2017) Chronic probiotic supplementation with or without glutamine does not influence the eHsp72 response to a multi-day ultra-endurance exercise event Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme 42:876-883 doi:10.1139/apnm-2017-0131
- McFarland LV, Evans CT, Goldstein EJC (2018) Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta- Analysis Frontiers in medicine 5:124 doi:10.3389/fmed.2018.00124
- Michalickova D et al. (2016) Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: a randomized, double-blind, placebo-controlled trial Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme 41:782-789 doi:10.1139/apnm-2015-0541
- Mörkl S, Butler MI, Holl A, Cryan JF, Dinan TG (2020) Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry Curr Nutr Rep 9:171-182 doi:10.1007/s13668-020-00313-5
- Muñoz-Quezada S et al. (2013) Competitive inhibition of three novel bacteria isolated from faeces of breast milk-fed infants against selected enteropathogens The British journal of nutrition 109 Suppl 2:S63-69 doi:10.1017/s0007114512005600
- Ouwehand AC (2017) A review of dose-responses of probiotics in human studies Benef Microbes 8:143-151 doi:10.3920/bm2016.0140
- Parker EA, Roy T, D'Adamo CR, Wieland LS (2018) Probiotics and gastrointestinal conditions: An overview of evidence from the Cochrane Collaboration Nutrition (Burbank, Los Angeles County, Calif) 45:125-134.e111 doi:10.1016/j. nut.2017.06.024
- Pham M, Lemberg DA, Day AS (2008) Probiotics: sorting the evidence from the myths Med J Aust 188:304-308 doi:10.5694/j.1326 5377.2008.tb01627.x
- Plaza-Diaz J, Gomez-Llorente C, Fontana L, Gil A (2014) Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics World J Gastroenterol 20:15632-15649 doi:10.3748/wjg.v20.i42.15632
- Pugh JN, Fearn R, Morton JP, Close GL (2018a) Gastrointestinal symptoms in elite athletes: time to recognise the problem? British journal of sports medicine 52:487-488 doi:10.1136/bjsports-2017-098376
- Pugh JN, Kirk B, Fearn R, Morton JP, Close GL (2018b) Prevalence, Severity and Potential Nutritional Causes of Gastrointestinal Symptoms during a Marathon in Recreational Runners Nutrients 10 doi:10.3390/nu10070811
- Pugh JN et al. (2019) Four weeks of probiotic supplementation reduces GI symptoms during a marathon race Eur J Appl Physiol doi:10.1007/s00421-019-04136-3
- Pugh JN, Wagenmakers AJM, Doran DA, Fleming SC, Fielding BA, Morton JP, Close GL (2020) Probiotic supplementation increases carbohydrate metabolism in trained male cyclists: a randomized, double-blind, placebo-controlled crossover trial American journal of physiology Endocrinology and metabolism 318:E504-e513 doi:10.1152/ajpendo.00452.2019
- Roberts JD, Suckling CA, Peedle GY, Murphy JA, Dawkins TG, Roberts MG (2016) An Exploratory Investigation of Endotoxin Levels in Novice Long Distance Triathletes, and the Effects of a Multi- Strain Probiotic/Prebiotic, Antioxidant Intervention Nutrients 8 doi:10.3390/nu8110733
- Rossi FW, Napolitano F, Pucino V, Capua G, Bianchedi D, Braconaro F, de Paulis A (2021) Drug use and abuse and the risk of adverse events in soccer players: results from a survey in Italian second league players Eur Ann Allergy Clin Immunol 53:37-42 doi:10.23822/EurAnnACI.1764-1489.163
- Sanchez B, Delgado S, Blanco-Miguez A, Lourenco A, Gueimonde M, Margolles A (2017) Probiotics, gut microbiota, and their influence on host health and disease Mol Nutr Food Res 61 doi:10.1002/mnfr.201600240
- Senok AC, Ismaeel AY, Botta GA (2005) Probiotics: facts and myths Clin Microbiol Infect 11:958-966 doi:10.1111/j.1469-0691.2005.01228.x
- Strasser B, Geiger D, Schauer M, Gostner JM, Gatterer H, Burtscher M, Fuchs D (2016) Probiotic Supplements Beneficially Affect Tryptophan-Kynurenine Metabolism and Reduce the Incidence of Upper Respiratory Tract Infections in Trained Athletes: A Randomized, Double-Blinded, Placebo-Controlled Trial Nutrients 8 doi:10.3390/nu8110752
- Stuempfle KJ, Hoffman MD (2015) Gastrointestinal distress is common during a 161-km ultramarathon Journal of sports sciences 33:1814 1821 doi:10.1080/02640414.2015.1012104
- Stuempfle KJ, Valentino T, Hew-Butler T, Hecht FM, Hoffman MD (2016) Nausea is associated with endotoxemia during a 161-km ultramarathon Journal of sports sciences 34:1662-1668 doi:10.1080/026404 14.2015.1130238
- Tscholl PM, Vaso M, Weber A, Dvorak J (2015) High prevalence of medication use in professional football tournaments including the World Cups between 2002 and 2014: a narrative review with a focus on NSAIDs British journal of sports medicine 49:580-582 doi:10.1136/bjsports-2015-094784
- West NP, Horn PL, Pyne DB, Gebski VJ, Lahtinen SJ, Fricker PA, Cripps AW (2014) Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals Clin Nutr 33:581-587 doi:10.1016/j.clnu.2013.10.002
- Zmora N et al. (2018) Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features Cell 174:1388-1405.e1321 doi:10.1016/j. cell.2018.08.041