It is widely acknowledged that protein is an essential component in muscle growth and repair. Our latest X-Change, written by Oliver Witard, takes an in-depth look at the three T’s (Type, Time and Total) of protein and how these impact improved body composition in an athletic population.
Read the full X-Change article here or download the PDF via the button below.
Practical Implications
- A sufficient intake of dietary protein is fundamental for promoting muscle hypertrophy and improving body composition in athletes and exercisers.
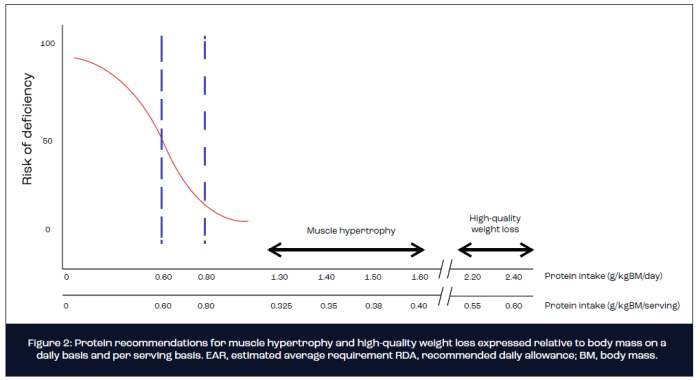
- A daily protein intake within the range of 1.3-1.7 g/kg body mass (BM)/day is recommended for athletes and exercisers with the goal of increasing or maintaining muscle mass and strength.
- A per meal protein serving in the region of 0.3-0.4 g/kgBM is recommended at 4 sittings across the day for the athlete or exerciser with the goal of muscle hypertrophy and/or exercise recovery.
- A daily protein intake in the region of 1.6-2.4 g/kg BM/day is recommended for athletes and exercisers with the goal of high-quality weight loss, meaning that the optimum per meal protein dose increases to 0.4-0.5g/kgBM at 4-5 sittings across the day.
- Dairy protein, and specifically whey, has been shown to promote the muscle anabolic response to resistance exercise to a greater extent than soy protein.
- Sustainable alternative protein sources derived from fungi, algae and insects have the potential to stimulate muscle anabolism and may be embedded into future protein recommendations for athletes and exercisers.
Introduction
Protein recommendations for athletes and exercisers is a key topic in the scientific field of sports nutrition that continues to rapidly evolve as new research is published and disseminated. A sufficient intake of dietary protein is fundamental to human health on multiple levels of physiology, including — but not limited to — (i) the structural (collagen and elastin) and contractile (actin, myosin and tropomyosin) properties of proteins, (ii) the role of proteins in transporting metabolites into tissues (e.g., glucose transporter 4 (GLUT-4) for glucose uptake into the muscle cell), (iii) communication via hormones and receptors, and (iv) the regulation of metabolic reactions and immune function (i.e., all enzymes and antibodies are proteins). In terms of athletic performance, and as a major biochemical constituent of skeletal muscle tissue (75% water, 20% protein, 5% divided between fat and glycogen), protein nutrition is synonymous with optimising muscle adaptation to exercise training. Accordingly, the aims of this brief article are three-fold. First, to summarise the latest evidence-based protein recommendations for promoting muscle hypertrophy and strength gains, with application to strength/power-based individuals. A discussion around protein recommendations for endurance-based individuals is beyond the scope of this article. Second, to present current protein recommendations for athletes and exercisers with the goal of improving body composition, i.e., the preservation of muscle mass and loss of fat mass. Finally, this article expands the Nutrition X-change Volume 10 by exploring the role of ‘sustainable’ alternative protein sources beyond plant proteins in promoting muscle hypertrophy and improving body composition in athletes and exercisers. To provide
theoretical context, the opening section of this article highlights commonly consumed protein-rich foods in the diet, and briefly explains the multiple metabolic fates of ingested protein, with emphasis on the metabolic basis of muscle hypertrophy.
Habitual protein intakes
The average daily protein intake in the UK is 88 grams for men and 64 grams for women, with the most consumed protein-rich foods being meat, cereals and cereal products such as bread, dairy products such as milk, yoghurt and cheese, as well as nuts and pulses, fish, and to a lesser extent eggs and egg dishes (National Diet and Nutrition Survey). Habitual protein intakes of athletic populations exceed the general population (Tipton and Witard, 2007). In this regard, the average protein intake reported across 9 studies of resistance-trained men was 2.0 g/kg body mass (BM) /day (Phillips et al., 2005), and in endurance-based athletes the average protein intake reported across 7 different studies was 1.8 g/kgBM/day (Tarnopolsky, 2004). On the other hand, the habitual protein intake of recreational exercisers has been estimated at 1.3 g/kgBM/day based on a large-scale study of ~900 young (19-30 years old) adults (Fulgoni, 2008).
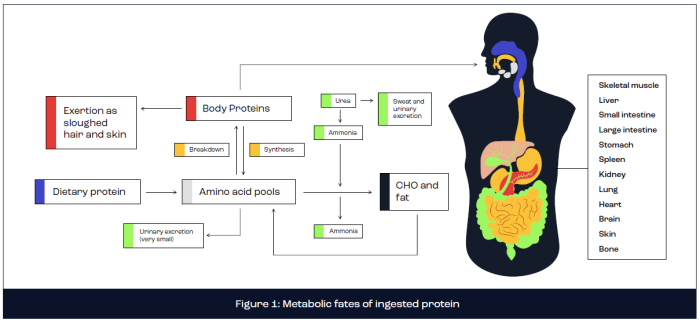
Metabolic fates of ingested protein
The metabolic fate of ingested protein is complex, encompassing digestion and absorption kinetics, anabolic and catabolic processes, energy production and excretion via urea production (Rennie and Tipton, 2000). In brief, dietary protein is digested in the stomach and small intestine via the action of enzymes secreted directly from the stomach (pepsin) or pancreas (trypsin, chymotrypsin, carboxypeptidase, elastase, aminopeptidase and dipeptidase). Following protein digestion, the constituent amino acids are absorbed across the intestine walls by active transport and delivered to the liver via the hepatic portal vein. The liver releases a large proportion of amino acids into the bloodstream for circulation to all body tissues, including skeletal muscle, where amino acids are metabolised. A small proportion of freely available amino acids are directly oxidised for energy production or are converted to glucose via gluconeogenesis or lipids via lipogenesis. However, the primary fate of amino acids concerns the provision of substrate for the synthesis of new functional body proteins, including skeletal muscle tissue. This remodelling of muscle tissue is fundamental for muscle adaptation, including muscle hypertrophy. Excess amino acids are excreted in the form of ammonia or urea (Figure 1).
Metabolic basis of muscle hypertrophy
In literal terms, hypertrophy is derived from the Greek word for ‘excess.’ It follows that (skeletal) muscle hypertrophy describes an increase in the size of existing muscle fiber cross sectional area that stems from an aggregate accumulation of muscle myofibrillar proteins (actin, myosin, tropomyosin and troponin) (Haun et al., 2019). Muscle proteins constantly undergo remodelling, meaning that old ‘damaged’ proteins are continuously broken down (termed muscle protein breakdown or MPB) and replaced with new muscle proteins via a process called muscle protein synthesis (MPS). Muscle protein is gained (hypertrophy) if rates of MPS exceed MPB, whereas muscle protein is lost (atrophy) if MPB exceeds MPS.
The importance of dietary protein for muscle hypertrophy is underpinned by the need to supply amino acids, i.e., the building blocks of muscle protein, as substrate for the working muscle following resistance exercise to accumulate muscle protein mass. While both MPS and MPB processes are modulated by protein ingestion, MPS is 4-5 times more responsive than MPB (Biolo et al., 1997). Accordingly, best practice nutrition guidelines for muscle hypertrophy in athletic populations are primarily based on protein recommendations for the stimulation of MPS. Over the past 30 years, the scientific literature has advanced our understanding of evidence-based protein recommendations to maximise muscle hypertrophy. This information has provided an important evidence base to help industry tailor protein-based products to consumer demands.
Protein recommendations for muscle hypertrophy
There is general consensus that the protein RDA (recommended daily allowance) of 0.8 g/kg BM/day is insufficient for athletic populations with the goal of maximising muscle hypertrophy in response to resistance training (Witard et al., 2019). Instead, a daily protein intake in the region of 1.3-1.7 g/kgBM/day is currently advocated. In absolute terms, this guideline equates to 104-136 grams of protein per day for the 80 kg individual. No clear benefits of protein intakes in excess of 1.6-1.7 g/kgBM/day are apparent with regards to muscle hypertrophy. This viewpoint is based on a recent systematic review and metaanalysis that concluded a protein intake in excess of 1.6 g/kgBM/day and as high as 2.2g/kgBM/day fails to augment resistance training induced gains in fat-free mass (FFM), at least in weight-stable trained individuals (Morton et al., 2018). Notwithstanding, and despite vehement concerns by non-experts regarding kidney problems and reduced bone mineral density, there is no substantive evidence that high protein diets are detrimental to athlete health. In fact, to the contrary, several studies have reported a positive relationship between dietary protein intake and markers of bone health (Shams-White et al., 2017).
The notion that protein recommendations are best expressed on a more refined per serving basis has garnered recent attention (Murphy et al., 2016). This idea is based on experiential evidence that multiple factors, including the per meal/serving dose, source (animal, plant or alternative), and timing (in relation to exercise) of ingested protein, as well as the coingestion of other nutrients (see Nutrition X-change volume 5) all modulate the response of MPS to ingested protein. As such, all factors should be considered alongside total daily protein intake when devising protein recommendations. Of these factors, the appropriate dose of protein to consume in a single serving after exercise is deemed the most important factor in modulating the response of MPS to ingested protein. In practice, many athletes and exercisers believe in the old “if a little is good for you, then a lot must be much better” mantra. However, based on scientific evidence, we (Witard et al., 2014) and others (Moore et al., 2009) have demonstrated that there is an upper limit to the amount of protein that can be effectively utilised for stimulation of MPS. For instance, we demonstrated that increasing the ingested dose of whey protein following leg only exercise from 20-40 g resulted in a marked increase in rates of amino acid oxidation and urea production and no statistically significant change in MPS (Witard et al., 2014). These data indicate that excess protein was merely broken down and oxidised or excreted in the form of urea rather than utilised to synthesis new muscle protein. A similar plateau in the response of MPS also was observed with ingested egg protein (Moore et al., 2009). Hence, it appears that only a finite amount of protein can be utilised by the muscle cell for the stimulation of MPS.
Based on findings from a series of tightly controlled laboratory studies, the optimum per serving dose of ingested protein expressed relative to body mass for maximal stimulation of MPS equates to ~0.3-0.4g/kg body mass (Moore et al., 2014). Hence, for an 80 kg athlete or exerciser with the goal of muscle hypertrophy, an absolute dose of 25-30 g of protein is recommended on 4 occasions over the course of a day, which translates to a protein intake of 1.2-1.6 g/kgBM/day. As a note of caution, most dose-response studies conducted to date have administered isolated proteins such as whey, egg or soy. The optimal per meal serving of protein-rich foods for the maximal stimulation of MPS remains largely unknown and warrants future investigation. It is conceivable that the optimal relative dose of protein-rich foods may approach 0.4-0.5 g/kgBM given the divergent digestion and absorption kinetics compared with intact proteins (Figure 2).
Protein source
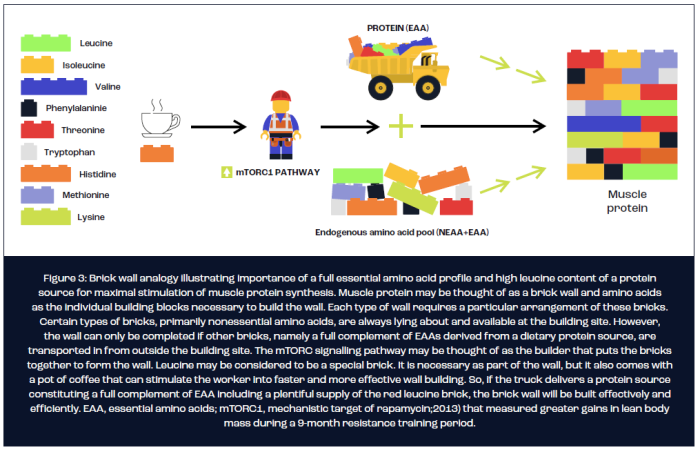
In terms of optimising the protein source for maximal stimulation of MPS, it is currently understood that three key nutritional factors determine the potential of a protein source to stimulate MPS. First, the digestibility of the protein source determines the bioavailability of amino acids as substrate for the synthesis of new muscle protein. Second, the rate at which amino acids appear in the circulation appears to act as a trigger, at least after exercise, for stimulating MPS. Finally, the protein source must constitute a complete profile of all nine essential amino acids (EAA), of which a high leucine content is necessary for a maximal MPS response. The brick wall analogy illustrated in figure 3 is often used to explain the importance of a protein source containing a full EAA profile and high leucine content for the maximal stimulation of MPS.
Amino acid composition and digestive properties can vary between different isolated types of intact proteins. The most common comparison of intact proteins within the scientific literature is between rapidly digested whey protein that is high in leucine content (~12.5% of total protein) and slowly digested casein protein that exhibits a relatively lower (~8.5% of total protein) leucine content. Studies in young (Tang et al., 2009) and older (Burd et al., 2012; Pennings et al., 2011) adults have consistently demonstrated a greater resting postprandial stimulation of MPS following ingestion of whey compared with casein protein. However, studies that compared the response of MPS or net muscle protein balance (NBAL; difference between MPS and MPB and thus indicative of the aggregate muscle protein anabolic response) to the post-exercise ingestion of whey and casein protein report equivocal results in young adults (Reitelseder et al., 2011; Tang et al., 2009; Tipton et al., 2004). Additionally, a recent study in young adults reported no difference in the chronic resistance training-induced increase in lean body mass (LBM) between whey and casein protein (Wilborn et al., 2013).
The discrepant findings between studies that fed intact whey and casein protein after exercise, at least in terms of acute measurements of MPS and NBAL, may be reconciled by general differences in study design. These differences include the form of intact protein ingested post-exercise (whey hydrolysate, whey isolate, micellar casein or calcium caseinate) and/or the time period over which MPS or NBAL was measured after protein ingestion. Micellar casein is insoluble and therefore is often treated with alkaline compounds such as calcium hydroxide to produce calcium caseinate. This treatment alters the digestion kinetics of casein, such that the rate of blood amino acid appearance with caseinate ingestion more closely mimics whey protein compared with micellar casein protein. Interestingly, acute studies that reported a differential post-exercise response of MPS between whey and casein protein ingestion administered micellar casein (Burd et al., 2012; Tang et al., 2009). Conversely, those studies that reported a similar post-exercise response of MPS or NBAL between whey and casein protein conditions administered calcium caseinate protein (Dideriksen et al., 2011; Reitelseder et al., 2011; Tipton et al., 2004). Taken together, this data suggests that ingesting the more rapidly absorbed caseinate elicits a greater anabolic stimulus compared with ingesting micellar casein. This insight supports the common perception that whey protein, due to amino acid composition (high EAA and leucine content) and rapid digestibility properties, is the highest-quality intact protein source popularised in protein supplements.
Variation in the time periods over which MPS or NBAL was measured also may explain the discrepant findings. An interesting observation is that studies reporting a greater response of MPS to whey compared with casein protein conducted measurements of MPS over a 4 h period or less after protein ingestion (Burd et al., 2012; Tang et al., 2009), whereas studies reporting no differences between whey and casein conditions obtained measurements of MPS or NBAL over 5 h or more (Reitelseder et al., 2011; Tipton et al., 2004). It is conceivable that “rapidly” digested whey protein stimulates a greater response of MPS in the early postprandial period (<4 h), however this advantageous “muscle protein anabolic response” is cancelled out in the late (>4 h) postprandial period by the more “slowly” digested casein. Accordingly, the timed ingestion of slowly digested casein protein before bedtime has been shown to increase the overnight stimulation of MPS in young and older adults (Groen et al., 2012; Res et al., 2012), and thus may be an effective strategy to increase muscle anabolism during overnight recovery.
Scientific studies also have compared the response of MPS to ingestion of whey and soy protein, which is relatively low in leucine content (~7.5% of total protein). A similar resting postprandial response of MPS to ingestion of whey and soy protein has been reported (Tang et al., 2009). However, acute metabolic data that demonstrate a greater postexercise response of MPS with whey compared with soy protein ingestion (Tang et al., 2009) are consistent with a tightly controlled longitudinal endpoint study of ~20 participants (Volek et al., 2013) that measured greater gains in lean body mass during a 9-month resistance training period with whey compared to soy protein supplementation. Future work is warranted to compare the response of MPS to ingestion of various isolated types of intact protein, both from animal (e.g., egg, fish, etc.) and plant (e.g., lentil, quinoa, maize, hemp, etc.) sources in resistance trained individuals.
Protein recommendations for improving body composition during weight loss
Weight control is a significant issue for many people, including athletes and exercisers. Athletic populations may desire weight loss for aesthetic reasons, to meet weight class requirements of a chosen sport (i.e., judo, boxing, mixed martial arts, etc), or to attain a better strength/power-to-body weight ratio for a performance advantage. High-quality weight loss describes the loss of fat mass while preserving, or even increasing, FFM (i.e., muscle tissue) during a voluntary, and typically short-term, period of energy restriction. Mechanistic studies have revealed the main driver of FFM loss during weight loss is a suppressed response of MPS with minimal changes in MPB (Pasiakos et al., 2010). This observation is intuitive given that MPS is energetically expensive, requiring a considerable ATP cost to drive this metabolic process. However, based on recent research, it is evident that manipulating the macronutrient content of an energy-restricted diet serves to modulate changes in body composition that result from a period of weight loss (Layman et al., 2003).
A growing body of evidence from both longitudinal (FFM as primary endpoint measurement) and acute metabolic (MPS as primary endpoint measurement) studies highlights the efficacy of increasing the protein content of an energy-restricted diet for ameliorating the loss of FFM during weight loss in athletes and exercisers (Longland et al., 2016; Mettler et al., 2010; Pasiakos et al., 2013; Pasiakos et al., 2015). For example, using a two-group parallel research study design, Mettler et al. (2010) measured changes in body mass, fat mass and FFM following 2 weeks of energy-restricted weight loss in resistance-trained males. The control group maintained their habitual protein intake at 1.0 g/kg BM/day, whereas the intervention group increased daily protein intake to 2.3 g/kg BM/day. On average, the control group lost 1.6 kg of FFM over 2 weeks of energy restriction, whereas FFM was essentially maintained (-0.3 kg) in the high protein group. Similar findings have been observed in studies of trained young females and male body builders. Moreover, a mechanistic study by Pasiakos et al. (2013) reported a suppressed postprandial response of MPS to 20 g of ingested milk protein following 21 days of energy restriction that was mitigated by increasing daily protein intake to 1.6 g/kg BM/day or 2.4 g/kg BM/day. Accordingly, a systematic review of all published studies concluded that a daily protein intake in the range of 1.8-2.7 g/kg BM/day is optimal for preserving FFM and maximising fat mass loss during energy restriction in athletic populations (Helms et al., 2014). Assuming 4-5 protein feeds per day, the target per meal dose of protein for stimulating MPS during energy restriction is increased to ~0.5g/kgBM. Hence, for the 80 kg athlete, this translates to ~40 g of protein per serving (Figure 2).
Future perspectives
To date, only a single study has investigated the impact of protein source on the response of MPS during energy restriction (Hector et al., 2015). In this study, thrice daily ingestion of 30g of whey protein stimulated a greater response of MPS over 1 day of energy restriction than a dose-matched soy protein regimen. These data are consistent with a directly comparable study conducted under conditions of energy balance whereby the response of MPS to 20g of ingested whey protein was greater than a dose-matched soy protein beverage (Tang et al., 2009). Other studies have reported a greater MPS response to the ingestion of milk vs. soy protein (Wilkinson et al., 2007), and casein vs. wheat protein (Gorissen et al., 2016). Taken together, these data are often used to support the notion that animal proteins stimulate a greater anabolic response than plant proteins, as mediated by the typically higher EAA content — specifically leucine that serves as a trigger for MPS — of animal proteins. As a note of caution, this conclusion is limited to the comparison of dairy and meat derived proteins (milk and beef) with only two plant proteins, namely soy and wheat. As detailed in The Nutrition X-change Volume 10, various other plant proteins exist, several of which exhibit a more favourable amino acid profile than soy and wheat. Hence, the field is just beginning to scratch the surface of the differential anabolic response to exercise with various types of protein.
Interest in alternative protein sources continues to gather momentum in terms of public health nutrition, primarily based on environmental concerns associated with the production of animal-based protein sources. In addition to plant proteins (reviewed in The Nutrition X-change Volume 10), alternative protein sources may be sourced from fungi, algae, insects and even lab-grown meat. Mycoprotein is a whole food produced from a fungus called Fusarium venenatum that boasts a favourable amino acid profile in terms of branched-chain amino acid content. Despite exhibiting slower digestion and absorption kinetics compared with milk protein, the ingestion of 70g of mycoprotein stimulated a robust MPS response to exercise in young men (Monteyne et al., 2020). Moreover, in older adults, the inclusion of mycoprotein as the primary protein source of a high protein (1.8 g/kgBM/day) vegan diet stimulated comparable daily rates of MPS vs. a protein-matched omnivorous diet. Equivocal findings have been reported for insect protein based on preliminary studies that administered mealworms to a cohort of healthy young adults. Whereas the ingestion of mealworm-derived protein augmented the acute response of MPS to resistance exercise (Hermans et al., 2021), this anabolic response did not translate to chronic improvements in FFM with mealworm ingestion compared with no supplementation (Vangsoe et al., 2018). Moving forward, there is clearly scope for future scientific studies to investigate the viability of other fungal-, algal- and insect-derived proteins for stimulating MPS during exercise recovery in athletic populations. As such, sport nutrition has a unique opportunity to lead by example in devising holistic protein recommendations that account for health, performance and sustainability outcomes.
Closing remarks
A primary function of dietary protein relates to the regulation of skeletal muscle mass, primarily via the stimulation of MPS. There is consensus that the protein RDA of 0.8g/kgBM/day grossly underestimates the protein needs for athletes and exercisers with the goal of muscle hypertrophy and/ or improved body composition. Instead, protein intakes of 1.3-1.7 g/kgBM/day are recommended for weight-stable athletes and exercisers with the training goal of muscle hypertrophy. Recommended protein intakes for athletes and exercisers with the goal of high-quality weight loss are up to 2-3 times greater than the current RDA at 1.6-2.4 g/kgBM/day. Claims that high protein diets, i.e., >2.0g/kgBM/day, are detrimental to kidney function and/or bone health are not substantiated by scientific evidence, at least in healthy athletic populations.
References
Biolo, G., Tipton, K.D., Klein, S., and Wolfe, R.R. (1997). An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. 273E122-E129.
Burd, N.A., Yang, Y., Moore, D.R., Tang, J.E., Tarnopolsky, M.A., and Phillips, S.M. (2012). Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br.J.Nutr. 1-5.
Dideriksen, K.J., Reitelseder, S., Petersen, S.G., Hjort, M., Helmark, I.C., Kjaer, M., and Holm, L. (2011). Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scand.J.Med.Sci.Sports 21e372-e383.
Fulgoni, V.L.,III. (2008). Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003-2004. Am.J.Clin. Nutr. 871554S-1557S.
Gorissen, S.H., Horstman, A.M., Franssen, R., Crombag, J.J., Langer, H., Bierau, J., Respondek, F., and van Loon, L.J. (2016). Ingestion of Wheat Protein Increases In Vivo Muscle Protein Synthesis Rates in Healthy Older Men in a Randomized Trial. J. Nutr. 146(9), 1651-1659.
Groen, B.B., Res, P.T., Pennings, B., Hertle, E., Senden, J.M., Saris, W.H., and van Loon, L.J. (2012). Intragastric protein administration stimulates overnight muscle protein synthesis in elderly men. Am. J. Physiol. Endocrinol. Metab. 302E52-E60.
Haun, C.T., Vann, C.G., Roberts, B.M., Vigotsky, A.D., Schoenfeld, B.J., and Roberts, M.D. (2019). A Critical Evaluation of the Biological Construct Skeletal Muscle Hypertrophy: Size Matters but So Does the Measurement. Front. Physiol. 10247.
Hector, A.J., Marcotte, G.R., Churchward-Venne, T., Murphy, C.H., Breen, L., von Allmen, M., Baker, S.K., and Phillips, S.M. (2015). Whey protein supplementation preserves postprandial myofibrillar protein synthesis during short-term energy restriction in overweight and obese adults. J. Nutr. 145(2), 246-252.
Helms, E.R., Zinn, C., Rowlands, D.S., and Brown, S.R. (2014). A systematic review of dietary protein during caloric restriction in resistance trained lean athletes: a case for higher intakes. Int. J. Sport Nutr. Exerc. Metab. 24(2), 127-138.
Layman, D.K., Boileau, R.A., Erickson, D.J., Painter, J.E., Shiue, H., Sather, C., and Christou, D.D. (2003). A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J.Nutr. 133411-417.
Longland, T.M., Oikawa, S.Y., Mitchell, C.J., Devries, M.C., and Phillips, S.M. (2016). Higher compared with lower dietary protein during an energy deficit combined with intense exercise promotes greater lean mass gain and fat mass loss: a randomized trial. Am. J. Clin. Nutr. 103(3), 738-746.
Mettler, S., Mitchell, N., and Tipton, K.D. (2010). Increased protein intake reduces lean body mass loss during weight loss in athletes. Med.Sci.Sports Exerc. 42326-337.
Moore, D.R., Churchward-Venne, T., Witard, O., Breen, L., Burd, N.A., Tipton, K.D., and Phillips, S.M. (2014). Protein Ingestion to Stimulate Myofibrillar Protein Synthesis Requires Greater Relative Protein Intakes in Healthy Older Versus Younger Men. J.Gerontol.A Biol. Sci.Med.Sci.
Moore, D.R., Robinson, M.J., Fry, J.L., Tang, J.E., Glover, E.I., Wilkinson, S.B., Prior, T., Tarnopolsky, M.A., and Phillips, S.M. (2009). Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am.J.Clin.Nutr. 89161-168. Morton, R.W., Murphy, K.T., McKellar, S.R., Schoenfeld, B.J., Henselmans, M., Helms, E., Aragon, A.A., Devries, M.C., Banfield, L., Krieger, J.W., and Phillips, S.M. (2018). A systematic review, meta-analysis and metaregression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 52(6), 376-384.
Murphy, C.H., Oikawa, S.Y., and Phillips, S.M. (2016). Dietary Protein to Maintain Muscle Mass in Aging: A Case for Per-meal Protein Recommendations. J. Frailty Aging 5(1), 49-58.
Pasiakos, S.M., Cao, J.J., Margolis, L.M., Sauter, E.R., Whigham, L.D., McClung, J.P., Rood, J.C., Carbone, J.W., Combs, G.F.,J, and Young, A.J. (2013). Effects of highprotein diets on fat free mass and muscle protein synthesis following weight loss: a randomized controlled trial. Faseb J. 27(9), 3837-3847.
Pasiakos, S.M., Margolis, L.M., and Orr, J.S. (2015). Optimized dietary strategies to protect skeletal muscle mass during periods of unavoidable energy deficit. Faseb J. 29(4), 1136 1142.
Pasiakos, S.M., Vislocky, L.M., Carbone, J.W., Altieri, N., Konopelski, K., Freake, H.C., Anderson, J.M., Ferrando, A.A., Wolfe, R.R., and Rodriguez, N.R. (2010). Acute energy deprivation affects skeletal muscle protein synthesis and associated intracellular signaling proteins in physically active adults. J.Nutr. 140745-751.
Pennings, B., Boirie, Y., Senden, J.M., Gijsen, A.P., Kuipers, H., and van Loon, L.J. (2011). Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am.J.Clin.Nutr. 93997-1005. Phillips, S.M., Hartman, J.W., and Wilkinson, S.B. (2005). Dietary protein to support anabolism with resistance exercise in young men. J.Am.Coll.Nutr. 24134S-139S.
Reitelseder, S., Agergaard, J., Doessing, S., Helmark, I.C., Lund, P., Kristensen, N.B., Frystyk, J., Flyvbjerg, A., Schjerling, P., van, H.G., et al. (2011). Whey and casein labeled with L-[1 13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am. J. Physiol. Endocrinol. Metab. 300E231-E242.
Rennie, M.J., and Tipton, K.D. (2000). Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu.Rev.Nutr. 20457-483. Res, P.T., Groen, B., Pennings, B., Beelen, M., Wallis, G.A., Gijsen, A.P., Senden, J.M., and van Loon, L.J. (2012). Protein ingestion before sleep improves postexercise overnight recovery. Med.Sci.Sports Exerc. 441560-1569.
Shams-White, M., Chung, M., Du, M., Fu, Z., Insogna, K.L., Karlsen, M.C., LeBoff, M.S., Shapses, S.A., Sackey, J., Wallace, T.C., and Weaver, C.M. (2017). Dietary protein and bone health: a systematic review and metaanalysis from the National Osteoporosis Foundation. Am. J. Clin. Nutr. 105(6), 1528-1543.
Tang, J.E., Moore, D.R., Kujbida, G.W., Tarnopolsky, M.A., and Phillips, S.M. (2009). Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 107987-992.
Tarnopolsky, M. (2004). Protein requirements for endurance athletes. Nutrition 20662-668.
Tipton, K.D., Elliott, T.A., Cree, M.G., Wolf, S.E., Sanford, A.P., and Wolfe, R.R. (2004). Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med.Sci.Sports Exerc. 362073-2081.
Tipton, K.D., and Witard, O.C. (2007). Protein requirements and recommendations for athletes: relevance of ivory tower arguments for practical recommendations. Clin.Sports Med. 2617-36.
Volek, J.S., Volk, B.M., Gomez, A.L., Kunces, L.J., Kupchak, B.R., Freidenreich, D.J., Aristizabal, J.C., Saenz, C., Dunn-Lewis, C., Ballard, K.D., et al. (2013). Whey protein supplementation during resistance training augments lean body mass. J.Am.Coll.Nutr. 32122-135.
Wilborn, C.D., Taylor, L.W., Outlaw, J., Williams, L., Campbell, B., Foster, C.A., Smith-Ryan, A., Urbina, S., and Hayward, S. (2013). The Effects of Pre- and Post-Exercise Whey vs. Casein Protein Consumption on Body Composition and Performance Measures in Collegiate Female Athletes. J.Sports Sci.Med. 1274-79.
Wilkinson, S.B., Tarnopolsky, M.A., Macdonald, M.J., MacDonald, J.R., Armstrong, D., and Phillips, S.M. (2007). Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am.J.Clin. Nutr. 851031-1040.
Witard, O.C., Garthe, I., and Phillips, S.M. (2019). Dietary Protein for Training Adaptation and Body Composition Manipulation in Track and Field Athletes. Int. J. Sport Nutr. Exerc. Metab. 29(2), 165-174.
Witard, O.C., Jackman, S.R., Breen, L., Smith, K., Selby, A., and Tipton, K.D. (2014). Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am.J.Clin.Nutr. 9986-95.







