Iron isn't something we talk about a lot, but a deficiency in this vital nutrient can be common place amongst the athlete population.
In our latest X-Change paper, co-written by Professor Peter Peeling, Dr. Alannah McKay, Mr Cory Dugan, Dr Rachel McCormick and Dr Marc Sim we take a detailed look at Iron and it's impact on the athlete.
Read the full X-Change article here or download the PDF via the button below.
Practical Implications
- Iron deficiency is a common nutrient disorder found in athlete populations, which can have negative implications to training adaptation and performance.
- Female athletes appear to be at greater risks of iron deficiency than their male counterparts.
- There are three core approaches to treating an iron deficiency, including dietary assessment and intervention with whole foods, oral iron supplementation or parenteral iron treatment.
- The choice of iron treatment will be dependent on the level of iron deficiency and the course of action should be made in consultation with a trained dietician or medical physician.
BACKGROUND
The dietary micronutrient, iron, significantly contributes to numerous key processes of relevance to exercise, which include red blood cell generation (and therefore oxygen delivery), cellular energy production, effective cognitive function and immune system modulation. Accordingly, healthy iron stores are imperative to athletes, yet iron deficiency is one of the most common nutrient disorders presenting in this population, with prevalence rates commonly reported at ~10% in males and ~30% in females [Sim et al., 2019]. However, smaller cohort studies of specific teams of athletes (i.e., a single national league women’s soccer team) have reported prevalence rates as high as 50% [Tan et al., 2012]. Given the importance to many exercise-relevant processes, we must question why are so many athletes iron deficient, and also, we must question why so many athletes are iron deficient, and also why females appear to be at greater risk compared to their male counterparts? The answers to these questions are complex, and there appear to be a multitude of reasons that can explain these problems. For instance, we know that having excess iron stores in the body can be toxic to our organs, and therefore the body regulates our dietary iron absorption in a homeostatic manner via the liver-produced peptide, hepcidin. In this process, when the body senses too much iron, hepcidin levels are increased to reduce iron absorption from the gut, and conversely, when iron levels are low, hepcidin levels are decreased to enhance our levels of iron uptake [Nemeth & Ganz, 2009]. The problem here is that a key driver to hepcidin elevation is the inflammatory cytokine, Interleukin-6, which we know is transiently increased after exercise, leading to an elevation in hepcidin levels some 3-6 h later [Peeling et al., 2009]. As such, it is likely that there are periods of reduced iron absorption in athletes during the (3-6 h) post-exercise period, which, over time, could contribute to their compromised iron stores [Peeling et al., 2009]. Add to this prospect that many exercise-induced mechanisms (sweating, GI-tract bleeding, hemolysis, etc.) can also negatively impact our iron stores (see [Peeling et al., 2008] for review), and we start to realise the multitude of factors that are at play. Notwithstanding, vegetarian athletes have the burden of reduced non-heme iron absorption from plant-based foods (as compared to heme iron from meat), female athletes incur menstrual blood losses in a cyclic fashion, and weight sensitive/aesthetic sports have the pressures of low energy intake which compromises the iron content of their food. Collectively, this results in a myriad of factors to consider. Ultimately, however, if we are working with athletes that present with compromised iron stores, we must first know how to recognise a problem exists, and then the strategies that may be used to treat it. Therefore, this paper attempts to draw these above concepts together, and to provide you with some contemporary approaches to addressing an iron deficit in athlete populations.
How do we monitor and define an iron deficiency?
To understand how iron deficiency is diagnosed, it is important to gain a basic understanding of the blood markers used to assess iron status. Currently, there is debate in the scientific literature as to the most effective blood markers that should be collected during routine blood screening to monitor iron status. However, current routine practice allows informed decisions to be made regarding iron status on the basis of an athlete’s serum ferritin (SF), their haemoglobin (Hb) and their transferrin saturation (TSAT). For context, SF provides an indication of the body’s iron stores, with its depletion being an early sign of iron deficiency. Such events indicate that the athlete’s iron requirements currently exceed iron availability, and therefore, a focus on iron intake is required. Left untreated, depleted iron stores can subsequently begin to impact red blood cell production, since iron is an essential component of Hb, whose role is imperative for the delivery of oxygen to the active muscle during exercise. One consequence of depleted iron stores is diminished red blood cell production, and over time, as red blood cell production is severely diminished from worsening iron deficiency (i.e., anemia), oxygen transport will inevitably suffer, and athletic performance will be negatively impacted. Finally, TSAT provides us a measure of the iron transport protein, transferrin, and its saturation with iron, which will be delivered around the body, predominately to the bone marrow for red blood cell production. Collectively, these three iron markers provide an indication of an athlete’s overall iron status, from which we can make informed decisions on any approaches required to treat a deficiency.
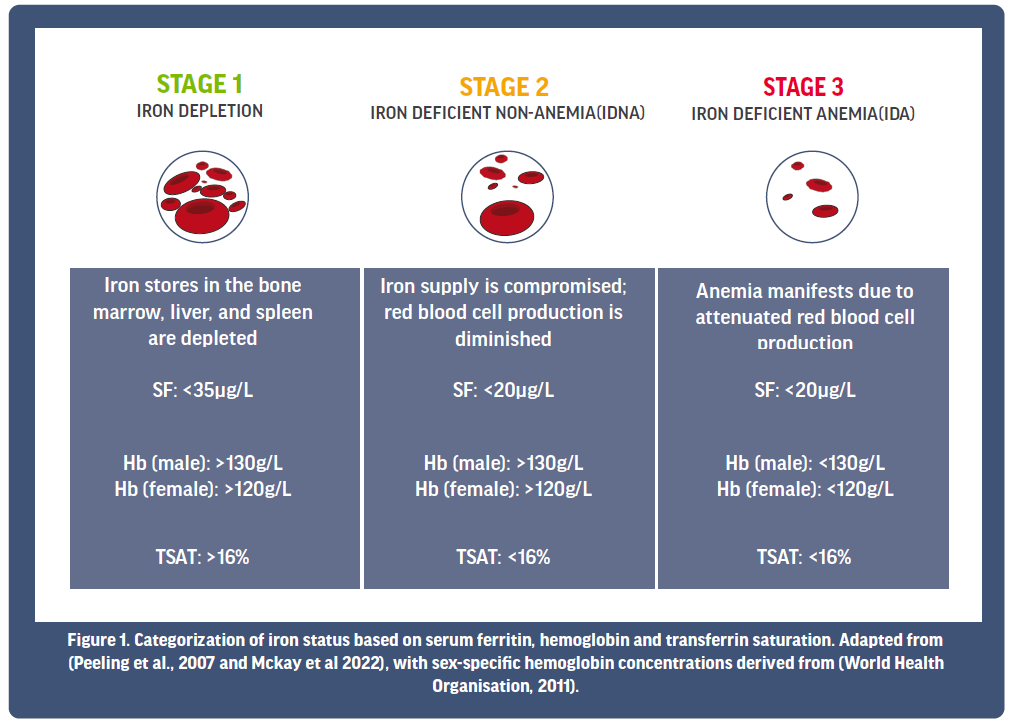
On the basis of these iron markers, current literature proposes that iron status for athletes can be classified into three main stages (see Figure 1). Stage 1 is characterised by a depletion in SF, although there is often no noticeable change to Hb or TSAT at this point. Left untreated, depleted iron stores can progress, causing red blood cell production to be diminished, and Stage 2 iron deficiency in the absence of anemia presents (IDNA). Progression of the condition from here then sees compromised red blood cell production, whereby Hb levels fall below a healthy status, indicating that Stage 3 iron deficient anemia (IDA) is now evident. Unsurprisingly, the degree by which iron deficiency affects performance and well-being is often related to its severity. For example, performance and health is likely to be most severely affected when iron stores are exhausted and red blood cell production/Hb are significantly compromised (e.g., Stage 3 IDA).
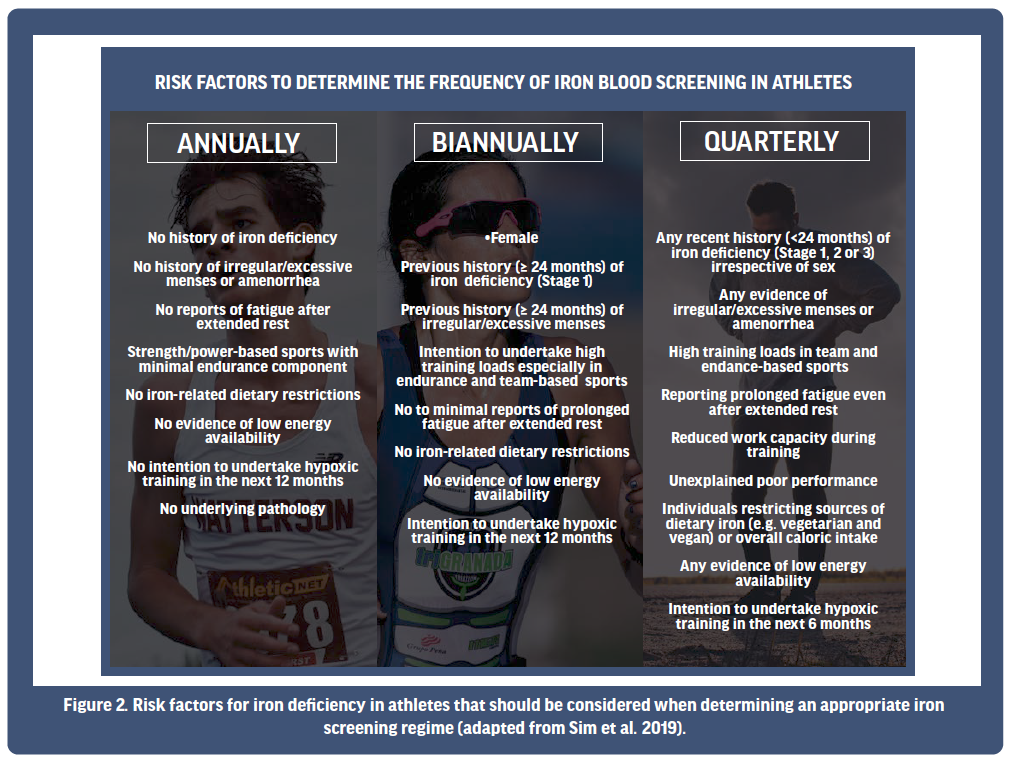
Given the aforementioned prevalence of iron deficiency in athlete populations, and the impact that such a deficiency might have on performance if left undiscovered or untreated, it is recommended that high-performance athletes consider, in consultation with their sports physician, at minimum, an iron screening on an annual basis [Sim et al., 2019, McKay et al., 2022]. However, if risk factors predisposing an athlete to being iron deficient are present, more frequent assessment (such as biannually or quarterly; Figure 2) might be considered. For example, females with any history of iron deficiency experiencing irregular/excessive menses are potential candidates for more regular iron screening, with medical practitioners likely considering the need to have their iron status assessed every six months. Alternatively, irrespective of sex, if iron deficiency has recently been recorded (i.e., in the past 2 years) in conjunction with unexplained prolonged fatigue and/or poor athletic performance, quarterly assessment might be warranted to assess the efficacy of any treatment undertaken to correct the problem. This is especially relevant to athletes predisposed to limited dietary iron availability, such as vegetarians and vegans, or those with low overall caloric intake. Considering such factors will likely facilitate prompt identification of iron deficiency in the early stages of the problem (e.g., Stage 1), thereby enabling timely intervention. To this end, practitioners might consider a focus on risk factors predisposing an athlete to iron deficiency as a guide to determining a suitable iron screening regime for each individual they work with.
Are there any special considerations for the female athlete?
As mentioned above, the prevalence of iron deficiency in female athletes is greater than that of their male counterparts. The most significant factor contributing to this sex difference is the loss of iron during menstruation, where between 5-40 mg of iron is lost each cycle [Harvey et al., 2005]. Accordingly, the menstrual status of an athlete can impact their ability to regulate iron. Heavy menstrual bleeding (HMB) is a condition defined as “excessive menstrual blood loss which interferes with a woman’s physical, social, emotional and/or material quality of life” [National Institute for Health and Care Excellence, 2021]. It is reported that HMB in athletes is highly prevalent [Bruinvels et al., 2016], and the high iron losses that occur can increase an athlete’s susceptibility to IDNA or IDA. Indeed, a recent survey of 1073 female marathon runners found that 35.5% had symptoms of HMB, of which, less than half had sought medical help for their symptoms (40%), and 41% had a prior history of IDA [4]. Use of the oral contraceptive pill (OCP) has been shown to minimise HMB in women [Lethaby et al., 2019], which is also likely to help decrease the risk of IDNA and IDA in these individuals. However, any such decision to utilise an OCP for the regulation of menstrual cycle should only be considered in conjunction with a trained medical physician. However, it is clear that identifying HMB and ensuring its effective management and treatment is important for maintaining optimal iron stores in the female athlete.
Conversely, amenorrhoea is a menstrual condition defined by the absence of ≥3 consecutive periods [Elliott-Sale et al., 2021]. Given that the iron losses usually occurring during menstruation are retained in amenorrheic athletes, amenorrhoea could be considered protective against iron deficiency [Petkus et al., 2017]. However, the reduced oestradiol concentrations seen in amenorrheic females increase the iron regulatory hormone, hepcidin [Yang et al., 2012], which, when elevated, reduces the ability to absorb iron from the diet [Nemeth & Ganz, 2009], likely resulting in an inability for an athlete to meet their daily iron requirements. Whether this impairment to iron absorption counteracts any potentially beneficial effect that amenorrhea has on iron metabolism is yet to be explored, and therefore should not be considered a positive prospect for reducing the prospects of IDNA or IDA.
Generally, any increased iron loss that occurs during menstruation needs to be compensated by an increased dietary iron intake so that iron balance is maintained. For this reason, the recommended dietary intake (RDI) for iron differs between sexes, where it is suggested that males and females need to consume 8 mg and 18 mg of iron each day, respectively [Thomas et al., 2016]. This can be difficult for many female athletes to achieve, particularly when balancing the requirement to meet other macro- and micronutrient targets. One issue that is highly prevalent in female athletes is low energy availability (LEA), whereby, energy intake is insufficient relative to energy expenditure, leaving inadequate energy available to support the normal physiological functions of the body [Mountjoy et al., 2018]. Common causes of LEA can be low caloric intake, which is often associated with low micronutrient intake, including that of dietary iron [Sim 2019]. Furthermore, excessive energy expenditure, or high training volume can also contribute towards a state of LEA, via exacerbated iron losses through the known exercise-induced avenues of iron loss (i.e., sweating, haemolysis, GI tract bleeding, etc. [Peeling et al., 2008]). Prolonged LEA can result in a range of negative health consequences, which collectively has been described as Relative Energy Deficiency in Sport (RED-S) [Mountjoy et al., 2018]. Iron deficiency has been proposed as one of the haematological outcomes that results from RED-S, and therefore, maintaining adequate energy availability is important to minimise the risk of IDNA or IDA ensuing.
So… How can we treat an iron deficiency?
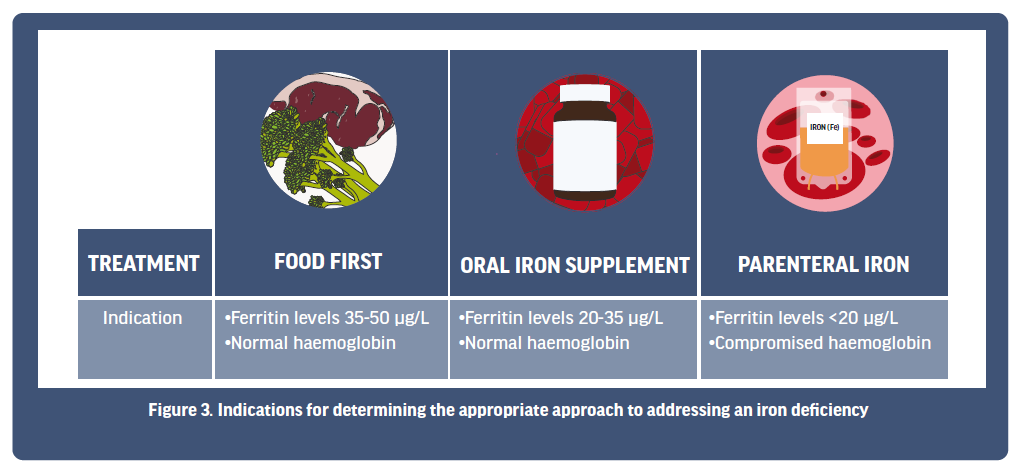
Currently, there are three main approaches to treating an iron deficiency, with a decision on the approach taken usually made relative to the results of an athlete’s iron screening (Figure 3). These three approaches can include (1) a food first response, where the overall intake of dietary iron is increased; (2) oral iron supplementation via iron tablets; or (3) parenteral iron injection or intravenous methods. These three approaches are somewhat aligned with the stages of iron deficiency, whereby athletes in Stage 1 iron depletion may first work with a dietician to see if their dietary iron intake can be improved from food first choices. Athletes in Stage 1 and/or Stage 2 IDNA might then progress to considering the use of an oral iron supplement to ensure an increase in overall iron intake. Finally, Stage 3 IDA athletes may work with their medical physician to decide if a more aggressive approach (parenteral) is needed to rapidly boost iron stores. Below, we will look at each approach in more detail.
1. Food first approaches
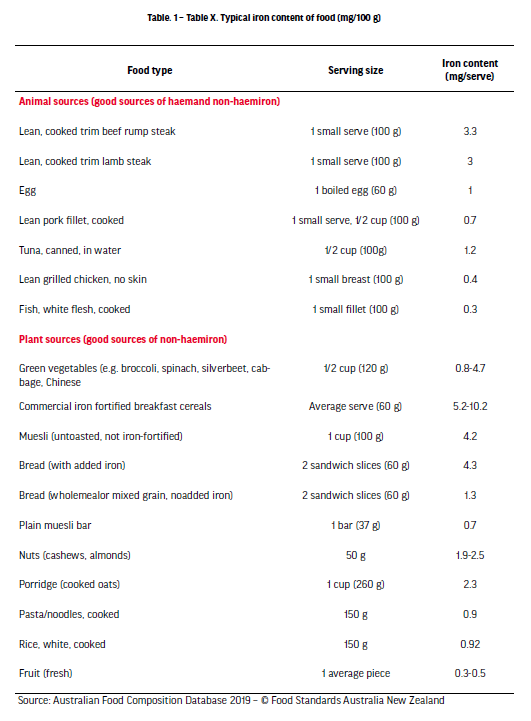
Given the body cannot endogenously synthesise iron, it is essential that athletes obtain and counteract their iron losses via daily dietary intake and absorption to maintain a healthy iron balance. Though iron balance is seemingly simple in principle, the low bioavailability of dietary iron, and the influences of exercise and other foods on iron absorption, collectively pose a challenge to athletes striving to attain their iron requirements. Increasing dietary iron intake is typically the initial and most conservative treatment for iron depletion [McCormick et al., 2020]. Upon the identification of negative iron balance, or iron depletion, it is strongly recommended that athletes consult with a qualified sports dietitian to identify and address any underlying dietary issues. For example, in the context of weight-class athletes or athletes with high training load, an intentional or unintentional low energy intake can simultaneously cause insufficient dietary intakes of several micronutrients, including iron. When assessing dietary iron intake, it is also important to consider that there are two forms of dietary iron that differ in bioavailability; haem iron, sourced from animal-based food, and non-haem iron, present in both plant and animal foods (See Table 1). Haem iron is absorbed considerably better than non-haem iron (15–35% and 2–20%, respectively), highlighting why vegetarian or vegan athletes are more susceptible to iron deficiency [Björn-Rasmussen et al., 1974; Monsen,1988]. Additionally, the absorption of non-haem iron is markedly influenced by several other dietary compounds commonly found in the diet; in some instances, acting to promote iron absorption, though more frequently, acting to inhibit iron absorption. Some common examples of food-constituents that decrease non-haem iron absorption are: phenolic compounds, including polyphenols and tannins contained in tea, coffee, and other plant foods; phytates, found in whole-grain cereals, legumes and nuts; and calcium, found in dairy food and green leafy vegetables [Brune et al., 1989; Hallberg et al., 1989; Hallberg et al., 1991]. Though not always practical, it is recommended to avoid an abundance of these inhibitors while consuming high-iron foods in order to minimise any negative impacts on iron absorption. On the contrary, ascorbic acid, more commonly known as vitamin C (50-100 mg), is the most powerful promoter of non-haem iron absorption and should therefore be consumed to coincide with iron intake in an attempt to maximise the potential for iron absorption [Diaz et al., 2003]. To further confound the low bioavailability of dietary iron, athletes may also need to consider the timing of their iron intake. The primary iron regulatory hormone, hepcidin, which acts to supress iron absorption, tends to show a diurnal effect, where it is lower in the morning as compared with the afternoon [Kenma et al., 2007]. Such a prospect suggests that iron absorption is likely going to be greater in the morning when hepcidin levels are at their lowest levels. Additionally, research consistently shows a 2- to 4-fold increase in hepcidin ~3 h following exercise, potentially reducing iron absorption in athletes within hours of finishing training [McCormick et al., 2019; Newlin et al., 2012; Peeling et al., 2009]. Interestingly, in a recent study that sought to investigate how the timing of exercise influenced iron absorption, researchers noted that the greatest absorption of iron was seen when athletes consumed a high dose of iron within 30 min of completing morning exercise (before hepcidin peaked after exercise) [McCormick et al., 2019]. This gave rise to a contemporary strategy of iron intake, whereby athletes are recommended to consume high iron-containing foods/supplements in the morning, rather than in the afternoon, and if exercising, to consume them as close to finishing exercise as possible, if not before [McCormick et al., 2020].
2. Oral iron supplementation
Whilst a food-first approach should always be advocated, there are a host of reasons why it may not always be possible for athletes to meet their iron requirements through diet alone (e.g., poor absorption, dietary preference, high training load, menstruation). Beyond dietary intervention, and in cases of IDNA athletes, oral iron supplementation should be considered in consultation with a doctor or trained sports dietician [McCormick et al., 2020]. Oral iron supplementation is the most widely used treatment to address iron deficiency. While there are many products on the market, a conventional supplementation regime consists of daily ferrous sulphate supplementation (~100 mg dose of elemental iron), usually found in combination with a source of vitamin C (i.e., Ferrograd C supplements contain 105 mg elemental iron and 500 mg of vitamin C), which typically increases an athlete’s iron stores by 30-50% over a 6–8-week period [Dawson et al., 2006; Hinton et al., 2000]. However, if athletes experience or cannot tolerate any of the frequently reported gastrointestinal side-effects (e.g., constipation, nausea, pain, diarrhea) associated with oral iron supplementation, particularly ferrous sulphate preparations, they should aim to adopt an alternate-day supplementation protocol (i.e., consuming ~100 mg of elemental iron every second day as opposed to daily). This strategy was recently shown to increase athletes’ iron stores comparably to daily supplementation, but with lower incidence of gastrointestinal side-effects, over 8 weeks in endurance runners [McCormick et al., 2020b]. However, if gastrointestinal symptoms persist, athletes may discuss with their practitioner the prospects of reducing their iron dosage (i.e., to a 60 mg dose – which may then take longer to have the same effect), swapping to an alternate oral iron preparation (i.e., enteric coated tablets or tablets combined with maltodextrin), or the prospects of by-passing the gut via consideration of intravenous iron replacement (the latter decision being made in consultation with the athlete’s medical physician).
3. Parenteral approaches
Generally reserved for later stage iron deficiency (i.e., IDA) where the body’s Hb levels have been compromised, parenteral iron therapies (injection of intravenous) are effective in by-passing the gut-related limitations of iron absorption, restoring iron levels within ~24 h [Auerbach & Deloughery, 2016]. To date, studies have reported 200-400% increases in SF levels from a 300 to 550mg dose of intravenous iron [Burden et al., 2015; Dawson et al., 2006; Garvican et al., 2014; Woods et al., 2014], with the carbohydrate encased intravenous compound, ferric carboxymaltose, having been shown to successfully treat cases where Hb is compromised (i.e., IDA [Lyseng-Williamson & Keating, 2009]), resulting in improved fatigue scores, mood states and exercise capacity [Favrat et al., 2014; Garvican et al., 2011; Woods et al., 2014].
However, research regarding the potential overall efficacy of parenteral iron therapy in athlete populations is somewhat contrasted based on the underlying levels of iron stores prior to treatment. For example, Burden and colleagues [2015] examined the effect of a single (500 mg) intravenous iron dose on repletion rates in IDNA male and female distance runners, with results showing that those undergoing the intravenous iron therapy had significant increases in SF and TSAT, but in the absence of effect on performance changes (V̇O2max, vV̇O2max, running economy, time to exhaustion) or on total haemoglobin mass [Burden et al., 2015]. Such conclusions have been corroborated in highly trained distance runners with clinically normal iron status (SF 30-100 μg/L) in the absence of compromised Hb levels [Woods et al., 2014].
In contrast to IDNA individuals, the benefits of parenteral iron therapy on iron status and performance has been positively demonstrated in individuals with the more severe IDA (for review see [Sim et al., 2019]). In states of IDA, both oxygen carrying capacity and tissue oxidative capacity are compromised, and therefore, treating IDA through intravenous iron therapy can rapidly improve iron stores, augmenting an athlete’s innate ‘toolbox’ to haematologically adapt to a training stimulus. Research undertaken in IDA athletes has therefore shown increases to haemoglobin mass, tissue oxidative capacity and V̇O2max from this intravenous approach [Hennigar, 2019]. This is evidenced in a case study of a 19-year-old, anaemic female endurance runner whose iron therapy involved an initial intramuscular iron injection of 100mg followed by two 100mg daily oral iron supplements for a 15-week period [Garvican et al., 2011]. Here, significant improvements in both iron status and haemoglobin mass were found across the 15-week period [Garvican et al., 2011], and at 8 weeks after the iron therapy commenced, a 3000m personal best time was ran. Accordingly, it seems that the positive impact of parenteral iron approaches to correcting iron stores are most effective in athletes with more severe levels of iron deficit (i.e., IDA), since the rapid effect of improving iron stores corrects the missing link in their ability to adapt (i.e., they can now improve Hb levels). However, given the stigma of athletes and needles, and the fact that increasing iron stores to unnecessarily high levels can be toxic, it is recommended that parenteral approaches to iron correction be reserved for severe cases of IDA, and that any decision to undertake this approach is taken in consultation with a trained medical physician.
Summary
Clearly, there is a complex interaction of factors that can act to impact an athlete’s iron status. Accordingly, at the elite level, having a strategic approach to monitoring iron status on an individual’s needs basis appears important. In the event of recognising an issue exists, understanding the stage of iron deficiency and the subsequent approach to treatment becomes valuable. In general, a food first approach to treatment would encourage a conversation between the athlete and their dietician to explore any dietary changes that might be considered to assist in rectifying the problem. If the diet and timing of food consumption relative to exercise appears adequate, or if feeding opportunities are restricted, oral iron supplements might be considered to improve iron stores over time. Of course, this can take 4-8 weeks to be effective, and there are possible negative gastrointestinal side-effects to take into account as the supplement period progresses. If both iron stores and Hb are compromised, and the time available for adaptation limited (i.e., by impending competition or training stimulus), consideration might be given to parenteral approaches to iron delivery, which by-pass the gut, rapidly improving iron stores and (if compromised) Hb. However, such decisions to undertake this form of treatment should be made in consultation with a trained medical physician. Ultimately, adequate iron stores are important to the health and wellbeing of an athlete, which can provide a ‘toolbox’ for haematological adaptation. Therefore, a strong understanding of the strategies we can use to address an iron deficiency when it arises can be used to help reduce the potential burden on an athlete.
Source: Australian Food Composition Database 2019 – © Food Standards Australia New Zealand
References
- Auerbach M, Deloughery T. Single-dose intravenous iron for iron deficiency: A new paradigm. Hematology Am Soc Hematol Educ Program. 2016; 2016(1): 57-66.
- Björn-Rasmussen E, Hallberg L, Isaksson B, Arvidsson B. Food iron absorption in man. Applications of the two-pool extrinsic tag method to measure heme and nonheme iron absorption from the whole diet. J Clin Invest. 1974; 53(1): 247-55.
- Brune M, Rossander L, Hallberg L. Iron absorption and phenolic compounds: importance of different phenolic structures. Eur J Clin Nutr. 1989; 43(8): 547-57.
- Bruinvels G, Burden R, Brown N, Richards T, Pedlar C. The prevalence and impact of heavy menstrual bleeding among athletes and mass start runners of the 2015 London Marathon. Br J Sports Med. 2016; 50, 566.
- Burden RJ, Pollock N, Whyte GP, Richards T, Moore B, Busbridge M, Srai SK, Otto J, Pedlar CR. Effect of intravenous iron on aerobic capacity and iron metabolism in elite athletes. Med Sci Sports Exerc. 2015; 47(7): 1399-407.
- Dawson B, Goodman C, Blee T, Claydon G, Peeling P, Beilby J, Prins A. Iron supplementation: oral tablets versus intramuscular injection. Int J Sport Nutr Exerc Metab. 2006; 16(2): 180-86.
- Diaz M, Rosado JL, Allen LH, Abrams S, García OP. The efficacy of a local ascorbic acid-rich food in improving iron absorption from Mexican diets: a field study using stable isotopes. Am J Clin Nutr. 2003; 78(3): 436-40.
- Elliott-Sale KJ, Minahan CL, de Jonge XAJ, Ackerman KE, Sipilä S, Constantini NW, Lebrun CM, Hackney AC. Methodological considerations for studies in sport and exercise science with women as participants: a working guide for standards of practice for research on women. Sports Med. 2021; 51: 843-61.
- Favrat B, Balck K, Breymann C, Hedenus M, Keller T, Mezzacasa A, Gasche C. Evaluation of a single dose of ferric carboxymaltose in fatigued, iron-deficient women–PREFER a randomized, placebo-controlled study. PloS one. 2014; 9(4): e94217.
- Garvican LA, Lobigs L, Telford R, Fallon K, Gore CJ. Haemoglobin mass in an anaemic female endurance runner before and after iron supplementation. Int J Sports Physiol Perform. 2011; 6(1): 137-40.
- Garvican LA, Saunders PU, Cardoso T, Macdougall IC, Lobigs LM, Fazakerley R, Fallon KE, Anderson B, Anson JM, Thompson KG, Gore CJ. Intravenous iron supplementation in distance runners with low or suboptimal ferritin. Med Sci Sports Exerc. 2014; 46(2): 376-85.
- Hallberg L, Brune M, Rossander L. Iron absorption in man: ascorbic acid and dose-dependent inhibition by phytate. Am J Clin Nutr. 1989; 49(1): 140-44.
- Hallberg L, Brune M, Erlandsson M, Sandberg AS, Rossander-Hultén L. Calcium: effect of different amounts on non-heme and heme iron absorption in humans. Am J Clin Nutr. 1991; 53(1): 112-19
- Harvey LJ, Armah CN, Dainty JR, Foxall RJ, John Lewis D, Langford NJ, Fairweather-Tait SJ. Impact of menstrual blood loss and diet on iron deficiency among women in the UK. Br J Nutr. 2005; 94(4): 557-64.
- Hennigar SR. Ironing out the Relation between Iron Supplementation and Exercise Performance in the Absence of Anemia. J Nutr. 2019; 149(2): 177-78.
- Hinton PS, Giordano C, Brownlie T, Haas JD. Iron supplementation improves endurance after training in iron-depleted, nonanemic women. J Appl Physiol. 2000; 88(3): 1103-11.
- Kemna EH, Tjalsma H, Podust VN, Swinkels DW. Mass spectrometry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications. Clin Chem. 2007; 53(4): 620-28.
- Lethaby A, Wise MR, Weterings MA, Bofill Rodriguez M, Brown J. Combined hormonal contraceptives for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019; 2(2): CD000154.
- Lyseng-Williamson KA, Keating GM. Ferric Carboxymaltose. Drugs. 2009; 69(6): 739-56.
- McCormick R, Moretti D, McKay AKA, Laarakkers CM, van Swelm R, Trinder D, Cox GR, Zimmerman MB, Sim M, Goodman C, Dawson B, Peeling P. The Impact of Morning versus Afternoon Exercise on Iron Absorption in Athletes. Med Sci Sports Exerc. 2019; 51(10): 2147-55.
- McCormick R, Sim M, Dawson B, Peeling P. Refining Treatment Strategies for Iron Deficient Athletes. Sports Med. 2020; 50(12): 2111-23.
- McCormick R, Dreyer A, Dawson B, Sim M, Lester L, Goodman C, Peeling P. The effectiveness of daily and alternate day iron supplementation in athletes with sub-optimal iron status (part 2). Int J Sport Nutr Exerc Metab. 2020b; 30(3): 191-96.
- McKay AKA, Sim M, Moretti D, Hall R, Stellingwerff T, Burden RJ, Peeling P. Methodological considerations for investigating iron status and regulation in exercise and sport science studies. Int J Sport Nutr Exerc Metab. 2022: 1-12. Epub ahead of print.
- Monsen ER. Iron nutrition and absorption: dietary factors which impact iron bioavailability. J Am Diet Assoc. 1988; 88(7): 786-90.
- Mountjoy M, Sundgot-Borgen JK, Burke LM, Ackerman KE, Blauwet C, Constantini N, Lebrun C, Lundy B, Melin AK, Meyer NL, Sherman RT, Tenforde AS, Klungland Torstveit M, Budgett R. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br J Sports Med. 2018; 52(11): 687-97.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management [online]. https://www.nice.org.uk/guidance/ng88/chapter/Context. 2021; Accessed: 23rd May 2022.
- Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009; 122(2-3): 78-86.
- Newlin MK, Williams S, McNamara T, Tjalsma H, Swinkels DW, Haymes EM. The effects of acute exercise bouts on hepcidin in women. Int J Sport Nutr Exerc Metab. 2012; 22(2): 79-88.
- Peeling P, Blee T, Goodman C, Dawson B, Claydon G, Beilby J, Prins A. Effect of iron injections on aerobic-exercise performance of iron-depleted female athletes. Int J Sport Nutr Exerc Metab. 2007; 17(3): 221-31.
- Peeling P, Dawson B, Goodman C, Landers G, Trinder D. Athletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormones. Eur J Appl Physiol. 2008; 103(4): 381-91.
- Peeling P, Dawson B, Goodman C, Landers G, Wiegerinck E, Swinkels D, et al. Effects of exercise on hepcidin response and iron metabolism during recovery. Int J Sport Nutr Exerc Metab. 2009; 19(6): 583-97.
- Petkus DL, Murray-Kolb LE, De Souza MJ. The unexplored crossroads of the female Athlete Triad and Iron Deficiency: A Narrative Review. Sports Med. 2017; 47(9): 1721-37.
- Sim M, Garvican-Lewis LA, Cox GR, Govus A, McKay AKA, Stellingwerff T, Peeling P. Iron considerations for the athlete: a narrative review. Eur J Appl Physiol. 2019; 119(7): 1463-78.
- Tan D, Dawson B, Peeling P. Hemolytic effects of a football-specific training session in elite female players. Int J Sports Physiol Perform. 2012; 7(3): 271-76.
- Thomas DT, Erdman KA, Burke LM. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J Acad Nutr Diet. 2016; 116(3): 501-28.
- Woods A, Garvican-Lewis LA, Saunders PU, Lovell G, Hughes D, Fazakerley R, Anderson B, Gore CJ, Thompson KG. Four weeks of IV iron supplementation reduces perceived fatigue and mood disturbance in distance runners. PLoS One. 2014; 9(9): e108042.
- World Health Organisation. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity [online]. https://apps.who.int/iris/handle/10665/85839. 2011; Accessed: 23rd May 2022.
- Yang Q, Jian J, Katz S, Abramson SB, Huang X. 17β-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology. 2012; 153(7): 3170-8.