How important are fats to your athletic performance? Our latest X-Change, written by Professor Don Maclaren, we'll guide you through this and how certain fats can be used as fuel in an athletic population.
Read the full X-Change article here or download the PDF via the button below.
Key Points
- Fats are the major storage form of energy in the human body (mainly as triglycerides in adipose tissue).
- Fats are a significant source of energy for endurance activities, derived mainly from breakdown of triglycerides to fatty acids by adipose tissue, and transported on albumin molecules in the blood to muscle.
- Fats are unable to supply energy during intense exercise bouts where CHOs are essential, although they supply energy for recovery between intense bouts of exercise.
- Feeding CHO before or during exercise impedes the ability to oxidise fats.
- Endurance training enhances the capability to oxidise fatty acids and thereby to conserve muscle glycogen.
- The maximal rate of fat oxidation (Fatmax) occurs at approximately 60% VO2max, although endurance training elevates this value.
- Ageing athletes have a lower propensity to oxidise fats, whilst female athletes have a greater propensity to oxidise fats than younger and male athletes respectively.
- When CHO intake is reduced to around 10% of intake over an extended period (say > 1 week or more), ketone body formation and enhanced fat oxidation occurs.
- ‘Fat loading’ is the concept of ingesting a high fat (70% energy intake) and low CHO (10% energy intake) over an extended period. Such a process significantly enhances the ability for fat oxidation, but is not likely to improve performance for high intensity bouts of exercise.
- ‘Fat loading’ with an ensuing short period of CHO loading may result in enhanced endurance performance.
- Caffeine ingestion increases fatty acid availability and may lead to an increase in fat oxidation, although this is not likely to directly enhance performance.
- Acute ingestion of Medium Chain Triglycerides (MCTs) fails to improve intense bouts of activity or moderate prolonged performance.
- There is some evidence that chronic ingestion of MCTs can lead to elevated cognitive function compared with placebo during exercise.
- Ketone body ingestion prior to or during exercise fails to enhance performance, although this area of research is relatively new.
Introduction
In a previous article the relevance of carbohydrate as an important source of energy for high-intensity work, as well as for prolonged activity, was demonstrated. Does that mean that fats are not relevant? What we do know is that fat sources are plentiful in the human body (even amongst the leanest individuals), but are unable to provide energy for muscles to operate at high intensity, although they are an important source of energy during prolonged activities. Furthermore, it is important to consider that there is a dependency on fat sources in the recovery period between high-intensity bouts. So, fats cannot be used anaerobically - such as for sprinting and power events - as they require aerobic processes to deliver energy.
Specifically, it is fatty acids that are the important sources of energy from lipids (where lipids is the overarching general term and fats or fatty acids are a sub-class of lipids i.e. all fats are lipids, but not all lipids are fats). There are plentiful supplies of fats even in the leanest person, and they can be found in adipose tissue as well as within intramuscular stores in the form of triglycerides (3 fatty acid molecules attached to a glycerol). Lipids are also important in so far as they are an integral part of the plasma membrane, and they also form the backbone of the sex hormones such as progesterone, oestrogen and testosterone.
Figure 1 demonstrates the importance of lipids as energy sources during exercise of increasing intensity. As exercise intensity increases (represented by percentage VO2max), note that the contribution of lipids reaches a peak at around 60 to 65% VO2max and thereafter decreases substantially – so much so that at 100% VO2max there is no contribution of lipid. This reflects the fact that lipids are unable to be used as an energy source for intense bouts of activity.
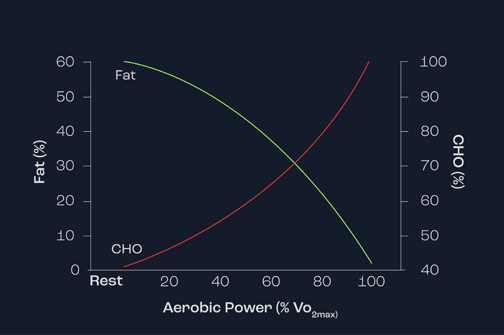
Figure 1. Relationship between exercise intensity and the use of carbohydrate and fat as energy sources.
On the other hand, with increasing duration of steady state exercise, lipids become more prominent in the later stages (Figure 2). It is common to observe the contribution of lipids to be in excess of 60% of the total energy used after 90 minutes of prolonged exercise. This is as a consequence of hormonal changes, resulting in the switch from carbohydrates to fats as a focus for energy. In particular, the hormone insulin acts as a regulator of fat release and, thereby, availability. When insulin is elevated (e.g. after a meal or a carbohydrate drink), there is an inhibition of fatty acid availability and a concurrent decrease in fat oxidation. However, when insulin levels in blood are low (e.g. after fasting or on a low CHO diet or during prolonged exercise), the inhibition on fat release is removed and greater fat oxidation occurs. Endurance-trained athletes are capable of using fats as their energy store to a greater extent than sedentary individuals or sprint-trained athletes. Indeed, they possess greater stores of intramuscular triglycerides.
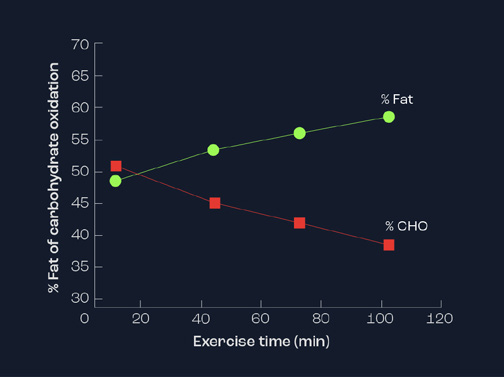
Figure 2. Relative contribution of carbohydrate and fat as energy source during steady state exercise lasting 100 minutes.
Figure 3 illustrates the contribution of carbohydrates and fats during exercise before and after a 12-week endurance training programme. N; note the use of fats from intramuscular triglyceride stores (IMTG) increases significantly and thereby results in reduced muscle glycogen use, i.e. muscle glycogen is spared.
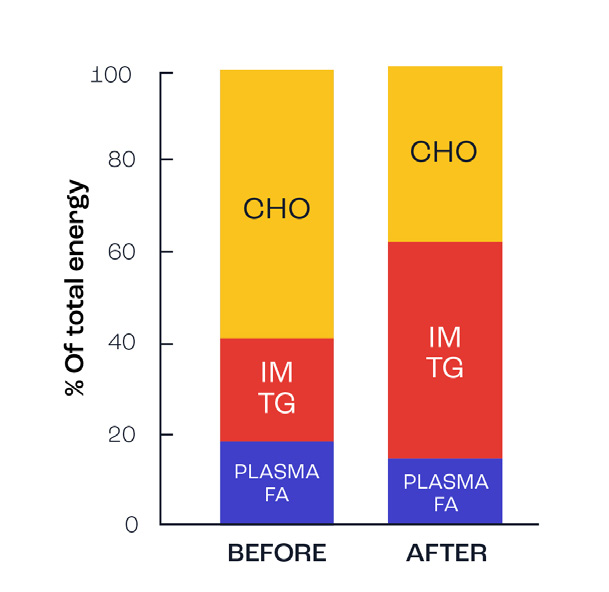
Figure 3. Change in fat oxidation after endurance training (adapted from Martin et al., 1993).
Skeletal muscle uses fatty acids (FA) as the energy source for oxidation within the mitochondria. The FAs are derived from adipose tissue triglyceride (TAG) stores and arrive at the muscle on albumin molecules, as well as from blood borne lipoproteins (derived in the liver after digestion). F) from where, they are transported across the muscle membrane by carrier proteins and can then either be stored as IMTG or oxidized within the mitochondria. It should be remembered that IMTG stores are also important, – notably for endurance trained athletes who possess greater levels of IMTG. Figure 4 highlights some aspects of the availability of – and use fat within muscle. Thus, far from being a useless source of energy, fats play an important role in exercise metabolism. Without fatty acids, the ability to exercise for prolonged periods of time would be difficult. On the other hand, it should be remembered that fats cannot be used during intense bouts of exercise, where carbohydrates are more likely to be used.
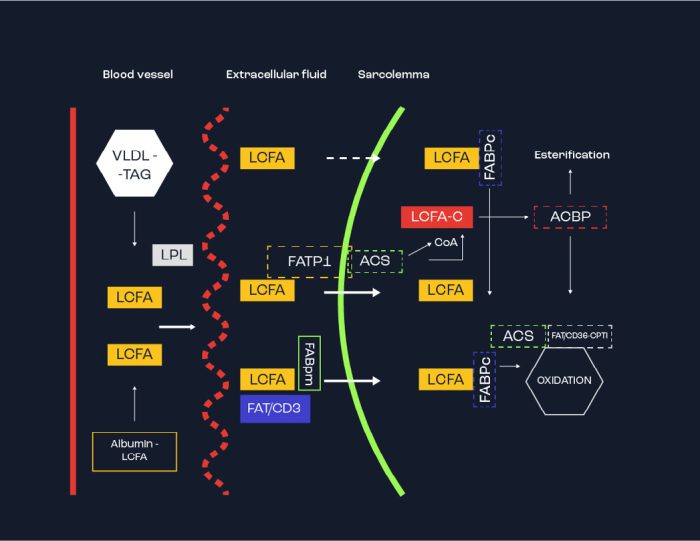
Figure 4. Schema of essential processes for uptake and oxidation of fats by a muscle cell. LCFA = long chain fatty acid either from adipose tissue via Albumin or on Very Low Density Lipoproteins (VLDL) arrive in the blood. The Fatty acids are liberated from the albumin whereas the Triglycerides (TG) release fatty acids due to an enzyme Lipoprotein Lipase (LPL). Three protein carriers on the muscle membrane that transport fats into the muscle are Fatty Acid Transport Protein (FATP), Fatty Acid Binding Protein (FABPpm) and CD36. Once inside the muscle, the fatty acids are carried by Fatty Acid Binding Protein (FABPO) and taken to the mitochondria for oxidation.
This article explores some of the literature concerning the use of fatty acids as an energy source during exercise, the inter-relationship between CHO intake and fat use, and the production and metabolism of ketone bodies before examining potential ergogenic ‘fat substances’ such as medium chain triglycerides (MCTs) and ketone body ingestion.
Fatty acids as an energy source during exercise
It must be emphasised that the quantity of fatty acid availability as an energy source far outstrips that of carbohydrates. F – for example, a 70kg person who has a 12% body fat should possess ~ 8.4kg of fat, and this is the equivalent of ~ 75,000 kcal energy! Likely total carbohydrate content, on the other hand, is maximally ~500g, which equates to 2,000 kcal of energy. So, fats are a virtually unlimited source of energy, whilst CHO are somewhat limited.
In order to illustrate the role of fats as an energy source during exercise, the classic work of Romijn et al. (1993) can be used (Figure 5). Participants exercised for up to 2 hours at 3 exercise intensities i.e. 25%, 65%, and 85% VO2max. Quite clearly, exercising at 85% was not possible for 2-h, but could be maintained for 30-min and hence there is no data for that intensity in the 90-120 min graph. The data highlights that fats are a predominant source of energy during the mild and moderate bouts of exercise, but not during intense exercise. Furthermore, that as the exercise duration increases beyond 30-min, more fats are used (see lower figure). Indeed, the major source of fat utilised is from the plasma fatty acids arriving from adipose tissue. Note the reduction in use of muscle glycogen at 65% between 90-120 min, as well as the significant use of muscle glycogen during the intense exercise bout.
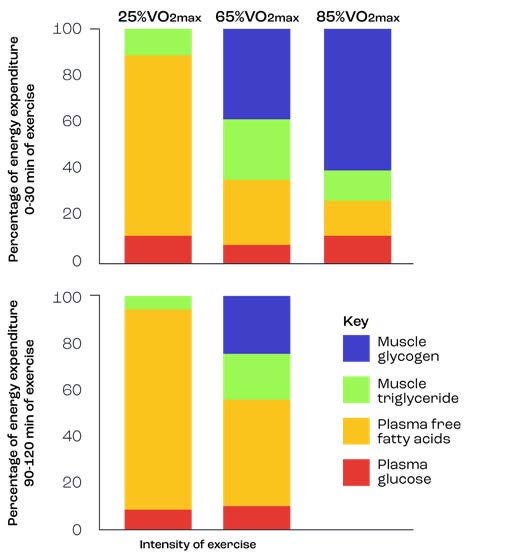
Figure 5. Substrate use at 3 varying exercise intensities (after Romijn et al., 1993) i.e. 25%VO2max (Mild exercise), 65%VO2max (Moderate exercise), and 85%VO2max (Intense exercise).
It therefore appears that mild/walking exercise employs fats for energy almost exclusively, that moderate exercise beyond 30-min uses fats predominantly, and that the energy for intense exercise is mainly furnished by muscle glycogen with little contribution from fats. For those wishing to undertake so-called ‘fat burning’ exercise, moderate to light exercise is preferable. However, since metabolism is elevated after exercise for some time, fats can continue to provide energy during recovery. Indeed, the more intense the exercise, the longer this recovery period and hence fats are ‘burned’/oxidised AFTER intense exercise rather than during intense exercise.
The concept of Fatmax
The amount of fat that can be oxidised during exercise is governed by the intensity of the exercise (although duration is an issue as well). The concept of Fatmax demonstrates this principle (Figure 6). If an individual is exercised at varying exercise intensities and the amount of fat oxidised is measured, the rate of fat oxidised increases to a peak and then decreases, following a bell-shaped curve.
The peak rate of fat oxidation invariably occurs at ~ 65% VO2max (~75% HRmax) and represents a fat oxidation rate of 0.4-0.6g/min. For endurance trained athletes, Fatmax occurs above this level i.e. 75-85% VO2max with a fat oxidation rate of 1.0-1.2g/min. A Fatmax of 0.4 to 0.6 g/min corresponds to 3.5-5.5 kcal/min of energy, whereas 1.0-1.2 g/min corresponds to 10.5 to 11.5 kcal/min; these rather low levels of energy production are not sufficient to be an exclusive energy source for exercise intensities required for sprinting or indeed relatively high intensify bouts (elite marathon runners, for example, run at nearly 20kph which demands energy at a rate of ~ 20 kcal/min). Clearly, fats are unable to provide energy for intense periods of activity, although the employment of fats helps to ensure the limited CHO stores are not depleted too early.
The reason for the decrease in fat oxidation with increases in exercise intensity is likely due to the enhanced use of muscle glycogen for energy at higher intensities and the concomitant increase in lactic acid production. The increase in lactic acid inhibits breakdown of fats in the adipose tissue and hence the availability of fatty acids is attenuated; – thus reduced fat oxidation.
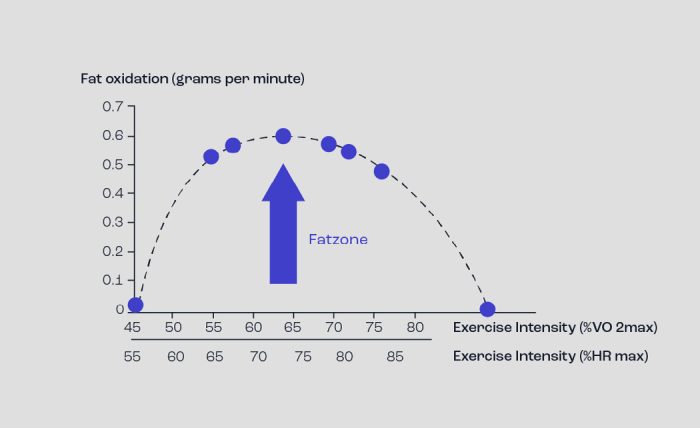
Figure 6. The concept of Fatmax. Note that as exercise intensity increases so does fat oxidation until a peak is achieved before fat oxidation then decreases.
Ageing and fat oxidation during exercise
As previously stated, endogenous fat is an important fuel for working muscles during endurance exercise. Two classical early studies demonstrated variances in fat metabolism between young and ageing participants during exercise
(Sial et al., 1996: 1998). In the first study (Sial et al., 1996) the effect of aging on fat and CHO metabolism during moderate intensity exercise was evaluated. The rate of appearance in plasma of glycerol (a measure of lipolysis in adipose tissue), fatty acid (FA), and glucose, as well as substrate oxidation, were assessed during 60 min of cycle ergometer exercise in six elderly (73 year old) and six young adults (26 year old) participants matched by gender and lean body mass.
The elderly group was studied during exercise performed at ~ 55% of VO2max, whereas the young adults were studied during exercise performed at the same absolute (i.e. same actual exercise load as the elderly on the cycle) and at a similar relative (i.e. ~55% VO2max) intensity as the elderly participants.
Fat oxidation during exercise was 25% lower in the elderly than in the young adults exercising at either the same absolute or similar relative intensities, whilst CHO oxidation in the elderly group was 35% higher than the young adults at the same absolute intensity but 40% lower when exercising at the same relative intensity. The rate of appearance of FA in plasma was 35% higher in elderly than in the young adults for the same absolute intensity but 35% lower than at a similar relative intensity. So, it appears that fat oxidation is lower while CHO oxidation is higher during moderate intensity exercise in the elderly. This shift in substrate oxidation was probably due to age-related changes in the capacity of skeletal muscle to oxidise fats because lipolytic rates (as assessed by glycerol in plasma) and FA availability for muscle were similar in elderly and young.
The slightly later study by Sial et al. (1998) explored the effect of a 16-week aerobic training programme on elderly (~74 year old) participants. Results demonstrated that endurance training increases fat oxidation without a significant change in lipolysis or FA availability during exercise. This once again confirming that the training-induced increase in fat oxidation during exercise is likely related to alterations in skeletal muscle fatty acid metabolism., but also that training adaptations in favour of increasing fat oxidation occurs in the elderly as well as the young.
Sex differences in fat oxidation during exercise
Several investigations conducted both in sedentary and recreationally- active individuals confirm greater reliance for fat oxidation in women compared with men during aerobic exercise (Horton et al., 1998; Cheneviere et al., 2011). Such evidence indicates that not only do women oxidize significantly more lipids than men, but they also use less carbohydrate to sustain such levels of exercise. Comparable findings have also been obtained with athletic, endurance-trained populations (Knechtle et al., 2004; Wallis et al. 2006).
These findings have been confirmed by a recent meta-analysis that concluded men display greater reliance on carbohydrates while women rely more on lipids to sustain moderate aerobic exercise, although, interestingly, this was not the case for athletic populations (Cano et al., 2022).
Carbohydrate intake and effect on fat oxidation
The oxidation of fats demands that blood insulin levels are reduced, since it is well understood that insulin supresses the breakdown of fatty acids from triglycerides in adipose tissue and thereby results in reduced fatty acids concentration in blood. As exercise progresses, there is an elevation of blood catecholamines (adrenaline and noradrenaline) which in turn inhibits insulin secretion. The result is that fatty acid availability increases, and this is what happens as exercise duration is longer. On the other hand, insulin secretion can be enhanced if CHO is ingested, – such as drinking CHO beverages before and/or during exercise. The net effect of CHO ingestion is reduced fat oxidation due to attenuated fatty acid levels.
In a variety of studies from my laboratory, we have observed that CHO ingestion during exercise reduces fat oxidation (Clarke et., 2011), that infusing glucose rather than a placebo (saline) reduces fat oxidation (MacLaren et al., 1996), and even feeding a CHO meal rather than a fat-containing meal prior to football simulation results in reduced fat oxidation (Hulton et al., 2013). These findings are consistent throughout the literature, and Figure 7 represents the response to CHO feeding during a 90-min (6 x 15-min) bout of simulated football exercise and effect on fat oxidation (Clarke et al., 2012).
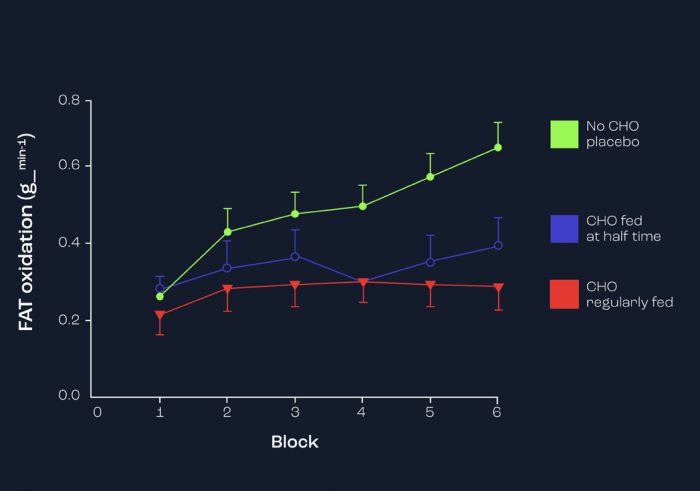
Figure 7. Effects of regular feedings of CHO vs placebo during 6 x 15-min bouts of simulated football running exercise on rates of fat oxidation.
An interesting study we reported with regards to a pre-exercise meal and cycling demonstrated that a high CHO breakfast had a significant negative impact on fat oxidation in a subsequent 90-min exercise bout, compared with a low CHO meal or in a fasted state (Whiteley et al., 1998) i.e. 0.46g/min, 0.54g/min, and 0.62g/min for high CHO, low CHO and no meal respectively. The fact that the no meal (i.e. 12-h fasted) provided the highest rate of fat oxidation was due to the fact that the 2 actual meals contained some CHO i.e. 20% for the low CHO and 86% for the high CHO meals. The meals contained typical breakfast items, and one conclusion that may be drawn from the data is that exercising after no meal (or possibly a meal with no CHO) results in greater fat oxidation than any meal with CHO. This may be useful for those who wish to undertake ‘fat burning’, where it could be suggested that ‘fat burning’ is best achieved in the morning when no breakfast is consumed.
So-called ‘Fat loading’
Since CHO feeding has been reported to have a significant positive impact on performance, does that imply that so-called fat feeding (or reduced CHO intake) results in a negative effect? A number of studies reported in the 1990’s actually observed a positive effect of short-term chronic (1-3 weeks) fat feeding on subsequent endurance performance when using endurance trained athletes (remember that endurance trained athletes can oxidise fats at higher exercise intensities). Muoio et al. (1994) used elite college middle distance runners who underwent a relatively high fat intake for 1 week and significantly enhanced time to fatigue, whilst Lambert et al (1994) employed elite road cyclists who also significantly improved time to fatigue following 2 weeks of dietary intervention. Indeed, Lambert et al. (2001) presented a follow-up study in which elite road cyclists undertook a 20-km time trial after 10-days on high fat or CHO diet with 3 days of high CHO feeding. A significantly faster time trial was attained with fat feeding with an additional 3 days of high CHO intake. Similar findings were observed by two other investigations where fat feeding was followed by 2 days of so-called CHO restoration days (Burke et al., 2000; Carey et al., 2001). In both of these studies time trial was significantly faster with fat feeding followed by 2-3 days of high CHO intake. So, it appears that short-term high fat feeding does not impair prolonged exercise performance, especially if there is a 1-3 days of CHO recovery. But what of long-term adaptation?
Phinney et al. (1983) undertook a study in which 5 endurance trained cyclists underwent 4 weeks of a low CHO high fat diet. After this time their fat oxidation rates were measured at 90g/h – this is significantly higher than the expected 60g/h reported in other studies of endurance athletes on a ‘normal’ diet. The practical implications of study findings are that the body can adapt to use fat as its primary fuel during submaximal exercise and so reduce the requirement for high rates of liver and muscle glycogen use. This can be appreciated by translating 90 g/h of fat oxidation to 810 kcal/hr of fat use in the keto-adapted athletes. If the total exercise energy expenditure is 930 kcal/hr, only 120 kcal/h is available from all other substrates (e.g., glucose, glycerol, amino acids). This demonstrates the degree to which these athletes are able to reduce the reliance on CHO dependence during endurance exercise, relying upon their greater reserves of body fat. More recently, a long-term study on ultra-endurance athletes was reported in which the participants were divided into a high CHO feeding (59% energy from CHO) and a high fat feeding (70% energy from fats) group for an average of 20 months (Volek et al., 2016).
After the 20-month time period those on the high fat intervention averaged a peak fat oxidation rate of 1.54g/min (92.4g/h), which was greater than the athletes on the high CHO plan (0.67g/min or 40g/h)). What was also of interest was that in spite of the high fat diet over this extended period, both resting and post-exercise muscle glycogen were similar between the 2 groups. No actual performance measures were undertaken, and so it is not possible to determine if such a long- term diet programme had a significant effect on performance. The rationale behind the long-term diet is that over an extended period of time, the body becomes in a state of ketosis i.e. where ketone body formation and use predominates. Ketone bodies (such as β-hydroxybutyrate and acetoacetate) are formed in the liver when the fatty acids in the blood are elevated (typically when fasted for a period of time or at least when there is a drastically reduced intake of CHO).
Figure 8 highlights the essence of ketone body formation in the liver. The ketone bodies then pass out of the liver and can be taken up by muscle as well as the brain as a preferred energy source. Both muscle and brain adapt to ketosis by reducing reliance on CHO use and instead use fatty acids and ketone bodies for energy.
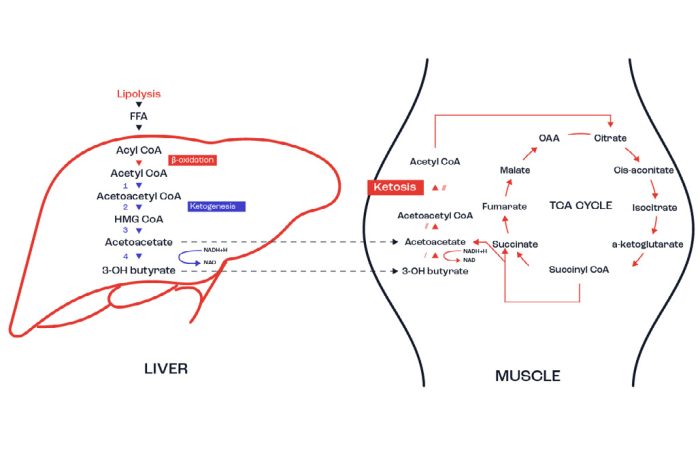
Figure 8. Ketone body formation and use in the human body.
Supplements and effects on fat oxidation
There have been a number of investigations exploring the use of supplements that elevate either fat availability or actually supply a ‘fat source’. Caffeine is an ergogenic supplement which is well recognised to increase fatty acid concentrations in the blood and thereby possibly lead to increased fat oxidation. On the other hand, MCTs and ketone body ingestion as ‘fat energy sources’ have also been examined.
Medium Chain Triglycerides (MCTs)
Medium chain triglycerides (MCTs) are mixed fatty acids with a chain length of between 6 and 12 carbon atoms. Naturally occurring sources of MCTs include coconut oil, palm kernel oil and breast milk. For the most part, commercially produced MCTs contain two predominant fatty acids in varying ratios; Caprylic Acid (C8) and Capric Acid (C10). MCTs are less common in Western diets, with the majority of the fatty acids consumed being long-chain fatty acids (LCFAs), i.e. containing more than 12 carbon atoms. These LCFAs are provided by animals/vegetable oils and fat sources which are vital for essential bodily functions. Despite not normally being present in typical western diets, MCTs are increasingly being incorporated into a growing number of food and nutrition plans due to their potential health benefits. Digestion of MCTs begins in the mouth by the enzyme lingual lipase which is present in saliva.
Figure 9 highlights that they are then hydrolysed in the stomach and intestine by pancreatic lipase into medium chain fatty acids (MCFAs) and monoglycerides. This is a faster and more complete process than that of long chain triglycerides (LCTs), with MCFAs being absorbed directly into the portal vein, bound to albumin, just minutes after ingestion. Moreover, absorption of MCTs may not require bile and lipase, and therefore, are directly absorbed by the enterocytes. Thus, they are unlike LCFAs, which are firstly incorporated into chylomicrons before being transported into the lymphatic system and taken to the liver.
The liver oxidises the majority of the MCFAs, leading to the production of ketones, predominantly β-hydroxybutyrate (βHB). As previously mentioned, the formation of βHB, as well as acetoacetate and acetone, is normally the result of a greater availability and oxidation of fatty acids in the liver due to an extended low carbohydrate intake, or during prolonged exercise. The ingestion of MCTs is more ketogenic (i.e. produces ketones more rapidly) in comparison to LCTs. For a thorough comparison of MCTs and LCTs, see Jeukendrup and Aldred (2004).
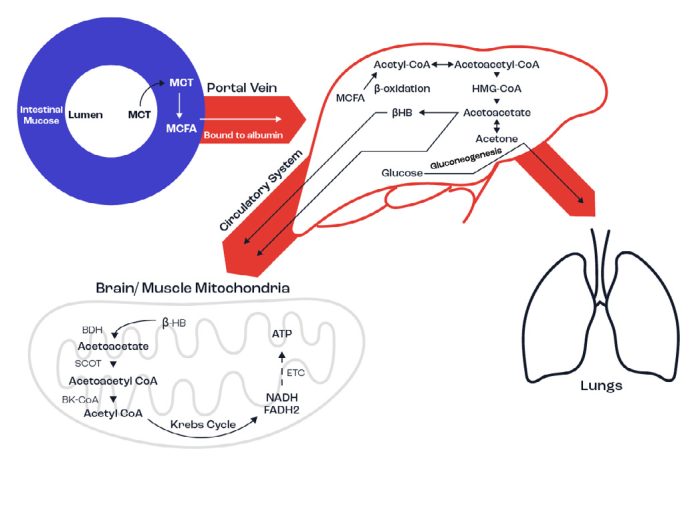
Figure 9. Schematic illustrating MCT metabolism in the body. Firstly, MCTs are hydrolysed in the small intestine by pancreatic lipase into medium chain fatty acids (MCFA) which are absorbed directly into the portal vein. The liver converts MCFA into the ketone Acetoacetate. This can then further breakdown into βHB and Acetone. Acetone is mainly excreted as a waste product through urine or CO2, but can also enter gluconeogenesis to produce glucose. Acetoacetate and βHB travel through the blood stream and enter the mitochondria of brain and muscle cells. Here, more Acetoacetate is generated from βHB which is then transformed into Acetoacetyl-CoA. Finally, acetyl-CoA enters the Krebs Cycle, producing energy. The ATP produced amounts to 23 molecules of ATP for each Acetoacetate molecule and 26 molecules for each βHB.
βHB is exported from the liver into the blood and converted in the mitochondria in muscle and brain cells into acetoacetate. The muscle utilises the ketone bodies as a direct source of energy, producing ~ 23-26 ATPs, whereas the brain can utilise βHB as an alternate energy source to glucose, which is normally the main fuel source. Additionally, the MCFAs that bypass metabolism in the liver can directly cross the blood-brain-barrier due to their relatively shorter carbon chain length and are oxidised in astrocytes: LCFAs are unable to do so. Therefore, MCTs provide additional fuel sources for the muscle and brain.
To date, only one study has reported a beneficial effect of acute MCT ingestion on performance (Van Zyl et al., 1996). In this study, 6 trained individuals ingested either a 10% CHO solution, a 4.3% MCT suspension or a combination of MCT plus CHO during 2 hours of submaximal exercise (60% of VO2max) which was immediately followed by a simulated 40km time-trial.
The amount of MCT ingested throughout the trial was 86g. Not only were the large doses of MCT apparently tolerated by their participants, but the addition of MCT to a CHO solution improved 40km time-trial performance by 2.5% compared with CHO alone. The authors attributed the enhanced performance to the larger doses of MCT ingested compared with previous studies. However, employing a similar experimental design during which MCT 85g was co-ingested with CHO, Jeukendrup et al. (1998) found no significant difference in the time to complete a set amount of work when MCT was added to CHO or when only CHO was consumed. In the latter study gastrointestinal cramping was more often reported when MCT were ingested than with carbohydrate. Jeukendrup et al. (1998) also reported impaired performance with MCT ingestion when compared with placebo. It appears that the ingestion of small (10 g/h) amounts of MCT have no major effects on FA metabolism, nor do they improve exercise performance, and that the ingestion of larger (30 g/h) amounts are likely to produce gastrointestinal problems in most athletes which would be expected to be detrimental to performance. Medium Chain Triglycerides supplementation may offer a possible solution to the cognitive decline linked to prolonged exercise. MCT supplementation has been shown to improve cognition at rest in individuals with reduced baseline cognition linked to age and cognitively debilitating diseases such as Alzheimer’s. These individuals benefit from MCT supplementation as the brain maintains its ability to utilise ketones, despite the decreased cerebral metabolism and/or cerebral insulin resistance due to age/disease inhibiting glucose metabolism.
Recently we demonstrated that MCT supplementation with a C8:C10 ratio of 30:70 enhanced cognition performance in tasks requiring working memory, executive function and task switching in a young and healthy cohort at rest after 3-4 weeks of supplementation (Ashton et al., 2021). It is therefore plausible that MCT supplementation may offset the cognitive decline associated with prolonged exercise.
Prolonged exercise has been linked to a decline in cognitive function due to a variety of factors, such as a drop in oxygen in the prefrontal cortex and an increase in stress hormones and neurotransmitters. Medium chain triglycerides (MCTs) may possibly offset this decline as they provide energy for the brain alongside promoting chronic physiological adaptations within the brain. Chronic MCT supplementation enhances resting cognitive performance and offsets the cognitive decline caused by a prolonged bout of exercise. In some cases, improvements in resting cognitive performance were maintained despite exercise.
Ketone body ingestion
Ketone bodies are elevated during periods of CHO restriction, starvation, as well as after some period of prolonged exercise. This is due to the fact that the limited CHO stores are nearing depletion and the alternative fat stores are required by muscle and brain. Recently, there has been an interest in the role of actually ingesting ketone bodies prior to exercise and the likely performance benefits, since the dietary strategies to increase endogenous ketone body availability (i.e., a ketogenic diet) require a diet high in lipids and low in carbohydrates for ~4 days to induce ketosis.
However, a high fat, low carbohydrate ketogenic diet may impair exercise performance due to reducing the capacity to utilize carbohydrate, which formsan important fuel for skeletal muscle during intense exercise.
Ketone body supplements, such as ketone salts and esters, have more recently emerged and may be used to rapidly increase ketone body availability without the need to adapt to a ketogenic diet. So what is the evidence (if any) that such an intervention is beneficial? As a result of prolonged fasting/starvation (~5 days), the rate of ketone body production results in a plasma concentration of ~7–10 mM, and beyond 5 days without food, plasma ketone body concentrations plateau and rarely exceed ~10 mM. Currently, commercially- available ketone body supplements provide 8–12 g of βHB per serving, whilst ketone esters have emerged as a more practical and applicable way to increase the availability of blood ketone bodies. Following ingestion, ketone esters are cleaved in the gut and absorbed into the circulation.
In comparison to starvation and/or a ketogenic diet which can take days to elicit an increase in ketone body concentrations, ingestion of ketone body supplements can rapidly increase plasma ketone body concentrations, reaching peak levels within 1–2 h. Plasma ketone body concentrations may increase to ~6 mM 1 h after ingestion of 400-600 mg of the ketone ester per kg body weight.
Having said that, a confounding variable is that relatively high plasma concentrations of BOH are more likely achieved when fasted and attenuated when the ketone body is ingested with a meal or a CHO drink. For greater detail, please consult Pinckaers et al. (2017) and Evans et al. (2022).
Caffeine
In a previous Xchange article, the benefits of caffeine ingestion on performance wereas reported (Clarke, 2022). This article also presented studies that clearly demonstrated the increase in plasma fatty acids after ingestion of caffeine as long as CHO wasn’t ingested at the same time. Figure 10 presents a finding from an early study (Giles & MacLaren, 1984) in which 6mg/kg body weight of caffeine 45-min before exercise not only increased plasma fatty acids but also promoted greater fat oxidation during exercise. It is understood that caffeine stimulates adrenaline secretion and so results in greater fatty acid availability through promotion of triglyceride breakdown (lipolysis) in adipose tissue. The greater fatty acid availability may be a potential factor in the positive effects of caffeine on endurance performance, although other factors are more likely. In spite of this, as well as the wealth of data illustrating the positive beneficial effects of caffeine on both intense and prolonged exercise activities, there is no firm relationship with improvements in performance due to caffeine and fat availability per se.
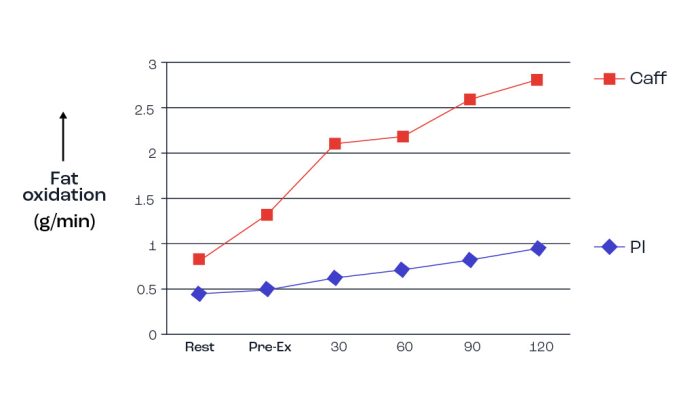 Figure 5. Substrate use at 3 varying exercise intensities (after Romijn et al., 1993) i.e. 25%VO2max (Mild exercise), 65%VO2max (Moderate exercise), and 85%VO2max (Intense exercise).
Figure 5. Substrate use at 3 varying exercise intensities (after Romijn et al., 1993) i.e. 25%VO2max (Mild exercise), 65%VO2max (Moderate exercise), and 85%VO2max (Intense exercise).
Conclusions
There have been a number of investigations exploring the use of supplements that elevate either fat availability or actually supply a ‘fat source’. Caffeine is an ergogenic supplement which is well recognised to increase fatty acid concentrations in the blood and thereby possibly lead to increased fat oxidation. On the other hand, MCTs and ketone body ingestion as ‘fat energy sources’ have also been examined.
References
Ashton JS, Roberts JW, Wakefield CJ, Page RM, MacLaren DPM, Marwood S, Malone JJ. (2021). The effects of medium chain triglyceride (MCT) supplementation using a C8:C10 ratio of 30:70 on cognitive performance in healthy young adults. Physiology &
Behavior 229:113252.
Burke, L. M., Angus, D. J., Cox, G. R., Cummings, N. K., Febbraio, M. A., Gawthorn, K., Hargreaves, M. (2000) Effect of fat adaptation and carbohydrate restoration on metabolism and performance during prolonged cycling. Journal of Applied Physiology, 89:2413–2421.
Cano A, Ventura L, Martinez G, Cugusi L, Caria M, Deriu F, & Manca A. (2022). Analysis of sex-based differences in energy substrate utilization during moderate-intensity aerobic exercise. European Journal of Applied Physiology 122:29–70.
Carey, A. L., Staudacher, H. M., Cummings, N. K., Stepto, N. K., Nikolopoulos, V., Burke, L. M., & Hawley, J. A. (2001). Effects of fat adaptation and carbohydrate restoration on prolonged endurance exercise. Journal of Applied Physiology, 91:115–122.
Chenevière X, Borrani F, Sangsue D, Gojanovic B, Malatesta D (2011) Gender differences in whole-body fat oxidation kinetics during exercise. Applied Physiology Nutrition and Metabolism. 36:88–95.
Clarke N. (2022). Caffeine’s impact on sporting performance. Nutrition Xchange volume 8.
Clarke, ND, Maclaren, DPM, Reilly, T, and Drust, BD (2011) Carbohydrate ingestion and pre-cooling improves exercise capacity following soccer-specific intermittent exercise performed in the heat. European Journal of Applied Physiology, 111:1447-1455.
Clarke, ND; Campbell, IT; Drust, B; Evans, L; Reilly, T; MacLaren, DPM (2012). The ingestion of combined carbohydrates does not alter metabolic responses or performance capacity during soccer-specific exercise in the heat compared to ingestion of a single carbohydrate. Journal of Sports Sciences. 30:699-708.
Clarke, ND; Campbell, IT; Drust, B; Evans, L; Reilly, T; MacLaren, DPM (2012). The ingestion of combined carbohydrates does not alter metabolic responses or performance capacity during soccer-specific exercise in the heat compared to ingestion of a single carbohydrate. Journal of Sports Sciences. 30:699-708.
Evans M, McClure TS, Koutnik AP, & Egan, B (2022). Exogenous Ketone Supplements in Athletic Contexts: Past, Present, and Future. Sports Medicine. 52:S25-67.
Giles D, MacLaren D (1984). Effects of caffeine and glucose ingestion on metabolic and respiratory functions during prolonged exercise. Journal of Sports Sciences 2, 35-46.
Hagberg JM, Seals DR, Yerg JE, Gavin J, Gingerich R, Premachandra B, Holloszy JO (1988) Metabolic responses to exercise in young and older athletes and sedentary men. J Appl Physiol 65(2):900–908.
Horton TJ, Grunwald GK, Lavely J, Donahoo WT (2006) Glucose kinetics differ between women and men, during and after exercise. Journal of Applied Physiology. 100:1883–1894.
Hulton, A T; Edwards, J P; Gregson, W; MacLaren, D; Doran, DA (2013) Effect of Fat and CHO Meals on Intermittent Exercise in Soccer Players. International journal of sports medicine 34:165-9.
Jeukendrup AE, Thielen JJ, Wagenmakers AJM, (1998). Effect of MCT and carbohydrate ingestion on substrate utilization and cycling performance. Am J Clin Nutr. 67:397-404.
Jeukendrup AE, & Aldred S, (2004). Fat supplementation, health, and endurance performance, Nutrition 20:678–688.
Knechtle B, Müller G, Willmann F, Kotteck K, Eser P, Knecht H (2004) Fat oxidation in men and women endurance athletes in running and cycling. International Journal of Sports Medicine. 25:38–44.
Lambert, E. V., Goedecke, J. H., van Zyl, C., Murphy, K., Hawley, J. A., Dennis, S. C., & Noakes, T. D. (2001). High-fat versus habitual diet prior to carbohydrate loading. Effects on exercise metabolism and cycling performance. International Journal of Sports Nutrition and Exercise Metabolism, 11: 209–225.
Lambert, E. V., Speechly, D. P., Dennis, S. C., & Noakes, T. D. (1994). Enhanced endurance in trained cyclists during moderate intensity exercise following 2 weeks adaptation to a high fat diet. European Journal of Applied Physiology and Occupational Physiology,
69:287–293.
MacLaren DPM, Reilly T, Campbell IT, & Hopkin C. (1999). Hormonal and metabolic responses to maintained hyperglycemia during prolonged exercise. Journal of Applied Physiology. 87: 124.
Phinney, S. D., B. R. Bistrian, W. J. Evans, E. Gervino, & G. L. Blackburn. (1983). The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation.
Metabolism 32: 769–7.
Pinckaers PJM, Churchward-Venne TA, Bailey D, & van Loon LJC. (2017). Ketone Bodies and Exercise Performance: The Next Magic Bullet or Merely Hype? Sports Medicine. 47:383–391.
Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, & Wolfe RR. (1993). Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. American Journal of Physiology. 265:E380-E391.
Sial S, Coggan AR, Carroll R, Goodwin J, Klein S (1996) Fat and carbohydrate metabolism during exercise in elderly and young subjects. American Journal of Physiology. 271:E983–E989.
Sial S, Coggan AR, Hickner RC, Klein S. (1998). Training-induced alterations in fat and carbohydrate metabolism during exercise in elderly subjects. American Journal of Physiology. 274:E785-90.
Van Zyl CG, Lambert EV, & Hawley JA. (1996) Effects of medium chain triglyceride ingestion on carbohydrate metabolism and cycling performance. J Appl Physiol : 80:2217-2225.
Volek JS, Freidenreich DJ, Saenz C, Kunces LJ, Creighton BC, Bartley JM, Davitt PM, Munoz CX, Anderson JM, Maresha,CM,
Lee EC, Schuenke MD, Aerni G, Kraemer WJ, Phinney SD. (2016). Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism Clinical and Experimental. 65:100-116.
Wallis GA, Dawson R, Achten J, Webber J, Jeukendrup AE (2006) Metabolic response to carbohydrate ingestion during exercise in males and females. American Journal of Physiology Endocrinology and Metabolism. 290: E708-E715.
Whitley HA, Humphreys SM, Campbell IT, Keegan MA, Jayanetti TD, Sperry DA, MacLaren DPM, Reilly T, and Frayn KN (1998). Metabolic and performance-related responses during endurance exercise after high-fat and high-carbohydrate meals. Journal of Applied Physiology 85, 418-424.










